Application of double-bond functionalized alpha-amino triethoxy silane in crosslinking or modification
A technology of functionalization of aminotriethoxysilane, which is applied in organic chemistry, chemical instruments and methods, dyeing organosilicon compound treatment, etc., and can solve the problems of high modifier cost, long modification time, and low efficiency , to achieve the effect of increasing hydrophobicity, improving compatibility, and changing hydrophilicity and hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
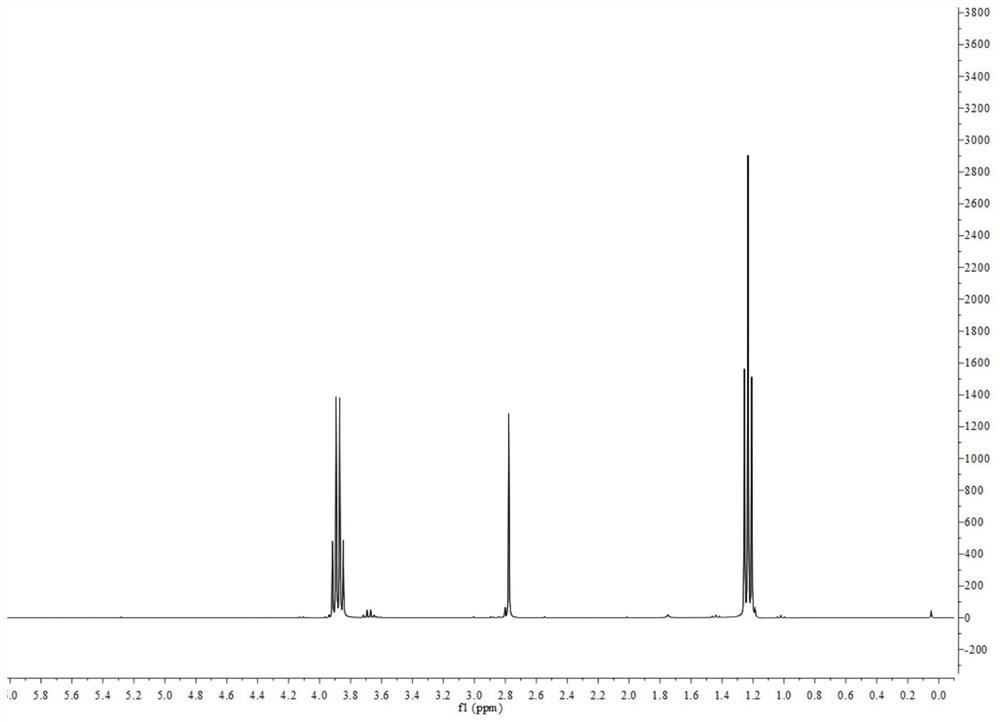
[0065] Preparation of chloromethyltriethoxysilane:
[0066] Add 36.036g of urea and 150ml of petroleum ether into a 250ml three-necked round-bottomed bottle, and the bottle mouth is connected with a spherical condenser and two constant-pressure dropping funnels. Under nitrogen protection, 27.642g of ethanol (with anhydrous treatment) and 30.000g of chloromethyltrichlorosilane were respectively added to two constant pressure dropping funnels. Under the protection of nitrogen, heat and stir the mixture of urea and petroleum ether to 65°C, then add ethanol and chloromethyltrichlorosilane synchronously at a rate of 1 drop / second; The reaction was stirred at ℃ for 5h. After the reaction, filter the reaction solution to remove insoluble viscous matter; heat the obtained filtrate to 90°C to remove petroleum ether and ethanol, and recover the obtained petroleum ether and ethanol; heat the remaining liquid to 70°C under a vacuum of 10mmHg , and distill off the transparent liquid to o...
Embodiment 1
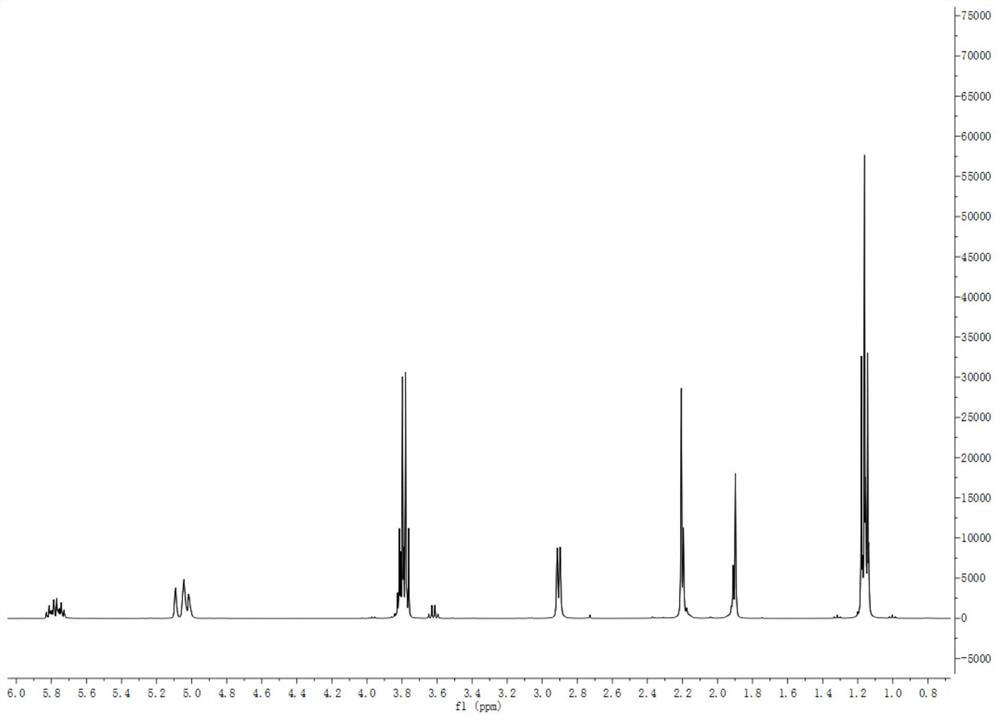
[0074] Application of α-(N-methyl-N-allyl)aminomethyltriethoxysilane as crosslinking agent for RTV silicone rubber
[0075] Take 3g of hydroxy silicone oil with a molecular weight of 3000, add 3ml of toluene to it, mix evenly, place it in a mold, and then use the hydroxyl and double bonds in the hydroxy silicone oil to functionalize the mole of ethoxy in α-aminotriethoxysilane The ratio is 1:1 Weigh 0.165g double-bond functionalized α-aminotriethoxysilane, dissolve it in toluene solvent, add it dropwise into hydroxyl silicone oil and stir quickly to mix evenly, at 15-16.5°C, 40-46% humidity Under the cross-linking curing.
[0076] The surface-drying time of the cross-linked mixed system after mixing hydroxy silicone oil-alkoxy silane was measured by finger touch method. The crosslinking curing time of α-(N-methyl-N-allyl) aminomethyltriethoxysilane p-hydroxyl silicone oil is
Embodiment 2
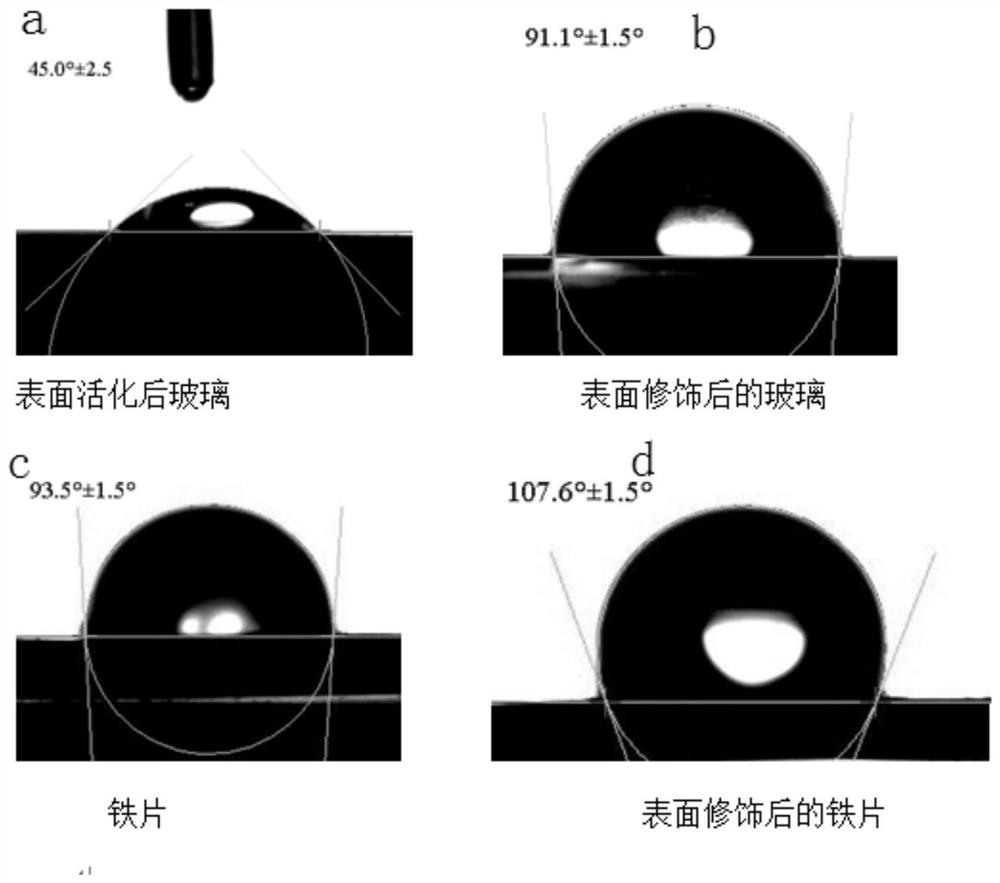
[0078] α-(N-methyl-N-allyl)aminomethyltriethoxysilane on SiO 2 surface modification
[0079] will settle SiO 2 Hydroxyl activation treatment was carried out by Piranha solution, and the hydroxyl content of silica after hydroxyl activation treatment was estimated by thermogravimetric method. According to characterization estimation, the hydroxyl content of silica after hydroxyl activation treatment was about 0.0032866mol / g.
[0080] Get six parts of 0.2g of silica after hydroxyl activation treatment, respectively add 5ml of anhydrous toluene as solvent, then add 0.65732mmol (0.162g) double bond functionalized α-aminotriethoxysilane respectively, 6 parts of mixture, According to the time of 5min, 10min, 30min, 1h, 1.5h, 2h, stop stirring and quickly remove the toluene solution by suction filtration, and then repeatedly wash the filtered SiO with a large amount of anhydrous toluene 2 . Then SiO2 is collected, and after drying, elemental analysis is carried out to detect its N ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com