Cracking agent suitable for direct amplification of DNA or RNA virus, kit and application of cracking agent and kit in PCR detection of virus
An RNA virus and lysing agent technology, applied in the field of DNA or RNA virus PCR detection kits, can solve problems such as long time consumption, cross-contamination, shortening extraction time, etc., and achieve the effect of improving detection efficiency and effective release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The lysing agent in this example is composed of 20mM Tris-HCL pH8.0, 150mM guanidine hydrochloride, 0.5% Tween 20, 0.5% acetylcysteine, 2.5mM EDTA, 500U / ml RNasin.
[0031] The fresh culture of high-concentration HBV virus (quantified by Roche’s COBAS AmpliPrep / COBAS TaqManHBV Test, version 2.0 kit) was serially diluted with commercially available negative and clear serum to obtain 10 6 、10 5 、10 4 、10 3 For samples with a concentration of IU / ml, mix the lysing agent and the diluted sample 1:1, treat at room temperature for 5 minutes, and take 5 μl for subsequent qPCR amplification.
[0032] HBV primer probe:
[0033] HBV-F: 5’-GTGTCTGCGGCGTTTTATCA- 3’
[0034] HBV-R: 5’-GACAAACGGGCAACATACCTT- 3’
[0035] HBV-P: 5'- CCTCTTCATCCTGCTGCTATGCCTCATC - 3' (markers: 5'FAM, 3'BHQ1)
[0036] components Volume (μl) / reaction 5XPCR buffer 5 dNTP (10mM) 0.5 Taq DNase (5U / μl) 0.6 Primer HBV-F (10 μM) 1 Primer HBV-R (10 μM) 1 Probe...
Embodiment 2
[0041] The lysis reagent in this example consists of 20mM Tris-HCL pH8.0, 100mM guanidine isothiocyanate, 0.5% Tween20, 0.5% acetylcysteine, 2.5mM EDTA, 500U / ml RNasin.
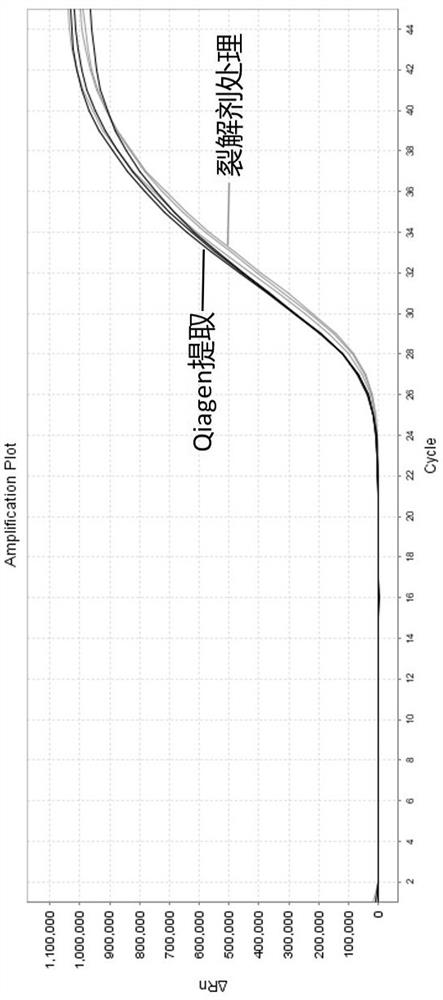
[0042] Dilute Coxsackie A16 virus (one of the main pathogens of HFMD) to 10 with a negative throat swab sample 5 、10 3 Copies / ml, treated with lysis reagent and extracted with Qiagen RNeasy Mini Kit, 10 5 Copies / ml set 3 duplicate holes, 10 3 Copies / ml set 20 duplicate wells, then take 5 μl as a template for RT-qPCR detection.
[0043] Coxsackie A16 virus primer probe:
[0044] Cox A16-F: 5'-GAACCATCACTCCACACAGGAG-3'
[0045] Cox A16-R: 5'-GTACCTGTGGTGGGCATTG-3'
[0046] Cox A16-P: 5'-CAGCCATTGGGAATTTCTTTAGCCGTG-3' (Markers: 5'FAM, 3'BHQ1)
[0047] components Volume (μl) / reaction 5XPCR buffer 5 dNTP (10mM) 0.5 Taq DNase (5U / μl) 0.6 MMLV enzyme (200U / μl) 0.1 Rnasin (200U / μl) 0.1 Primer H1N1-F (10 μM) 1 Primer H1N1-R (10 μM) 1 Probe H1N1-P (10 μM)...
Embodiment 3
[0052] The lysing agent in this example consists of 20mM Tris-HCL pH8.0, 100mM guanidine hydrochloride, 0.5% Tween 20, 0.5% acetylcysteine, 2.5mM EDTA, 500U / ml RNasin.
[0053] Prepare 15 positive samples from throat swabs of Influenza A (H1N1) virus, treat them with lysing agent and extract with QiagenRNeasy Mini Kit in parallel. RT-qPCR detection for the template.
[0054] Influenza A H1N1 Influenza Virus Primer Probe:
[0055]H1N1-F:5'-GGACTGCAGCGTAGACGCTT-3'
[0056] H1N1-R:5'-CATCCTGTTGTATATGAGGCCCAT-3'
[0057] H1N1-P: 5'-CTCAGTTATTCTGCTGGTGCACTTGCCA-3' (Tag: 5'FAM, 3'BHQ1)
[0058] The RT-qPCR reaction system and procedure are the same as in Example 2.
[0059] The test results are shown in the table below:
[0060]
[0061] The detection results showed that 15 cases of influenza A H1N1 influenza virus throat swab positive samples were detected by both methods, and the Ct value showed that the detection ability of the lysing agent of the present invention to tre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com