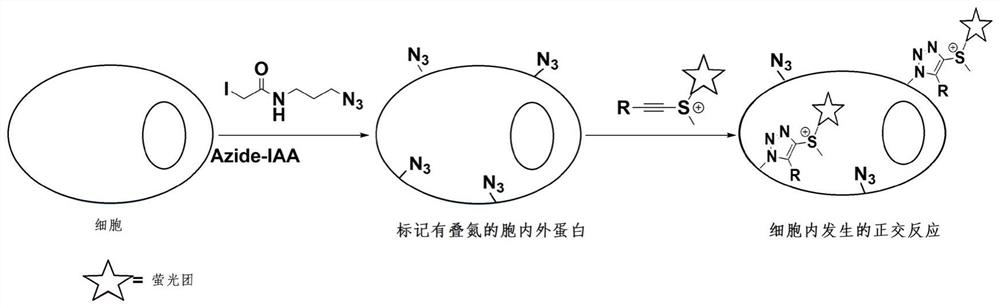
Protein labeling method for acetenyl sulfosalt click reaction
A click reaction and protein labeling technology, applied in the fields of chemical biology and biology, can solve the problem that probes are difficult to be absorbed by cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Example 1: Synthesis of ethynylsulfur salt and efficient click reaction with azide compound to generate 1,5-triazole cycloaddition product.
[0029] Based on previous literature, we synthesized a series of ethynylsulfonate substrates via a one-pot method. Synthesis of Ethynyl Sulfide from Dimethyl Sulfoxide The reaction equation is as follows:
[0030]
[0031] In a 100 ml three-necked flask, sulfoxide (5 mmol, 1.0 equivalent) was dissolved in 40 ml of dichloromethane with nitrogen gas exchange, cooled to -50 ° C, and trifluoromethanesulfonic anhydride (5 mmol, 1.0 equivalent) was stirred at this temperature for 1 hour, trimethylsilyl alkynyl (5 mmol, 1 equivalent) was dissolved in 5 ml of dichloromethane, and added dropwise to the reaction solution. Slowly raise the temperature to -15°C and stir for 6 hours. After the reaction, the solvent is spin-dried in vacuo, and the crude product is recrystallized to obtain products 2a-2n.
[0032] Under the same experiment...
example 2
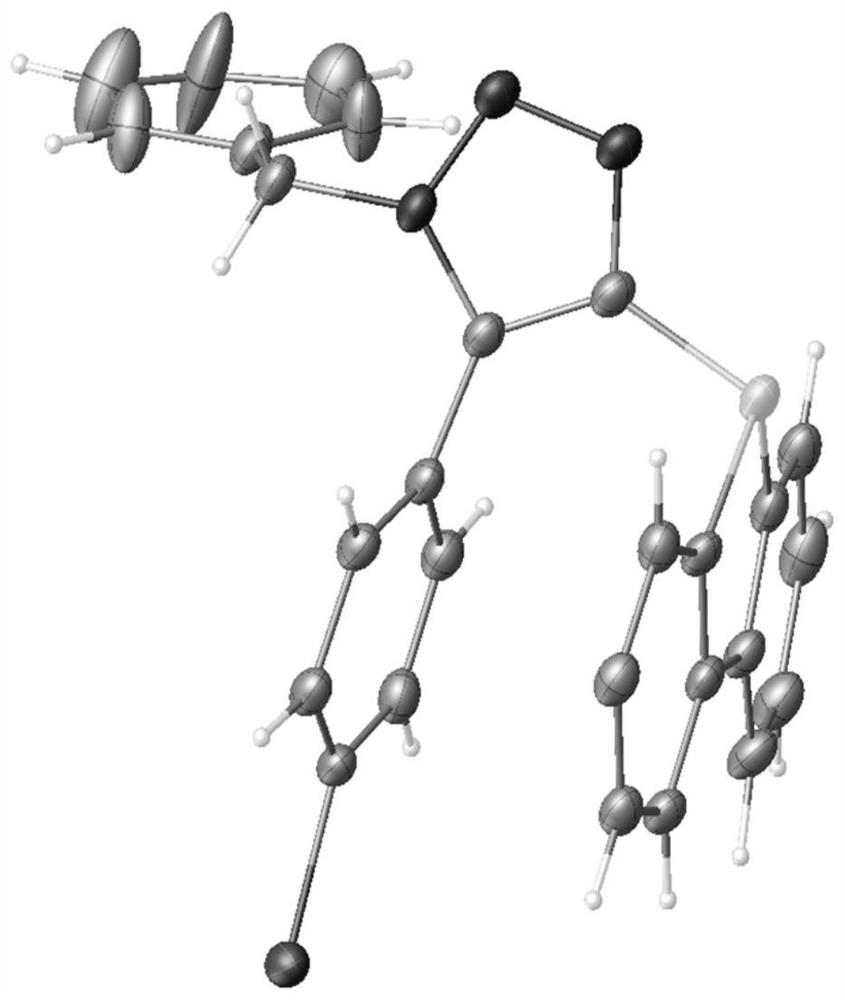
[0041] Example 2: X-ray diffraction analysis of 3g of 1,5-triazole cycloaddition single crystal product.
[0042] In order to fully clarify the product structure, we have carried out careful nuclear magnetic resonance (NMR) experiments and X-ray diffraction analysis of 3g single crystals to the product 3g prepared by the method of Example 1, as figure 2 shown. The distances of C-N, C-C and N-N bonds in the 1,5-triazole cycloaddition structure are consistent with the results for five-membered rings.
example 3
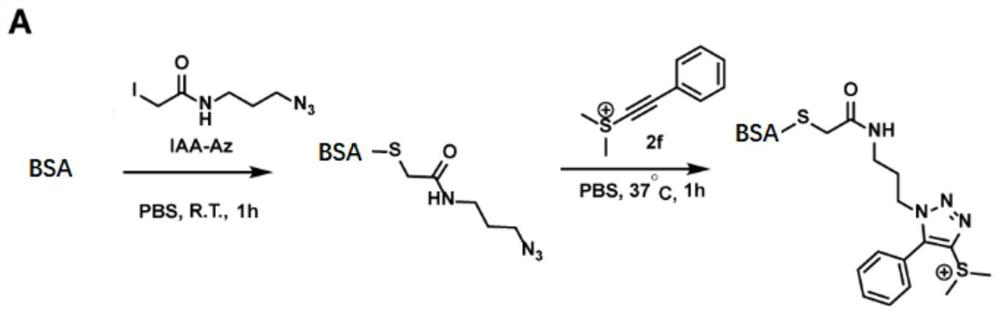
[0043] Example 3: Identification of acetylene sulfide salts in protein labeling by secondary mass spectrometry.
[0044] After confirming that ethynyl sulfide easily undergoes a click reaction with azide, we performed protein labeling experiments. In this labeling reaction, 2.5 mg / mL bovine albumin (BSA) was incubated with 1 mM azide alkylating reagent IAA-Az at room temperature for 1 hr to install a bioorthogonal N 3 group, and then incubated with ethynylsulfonate 2f only in phosphate-buffered saline PBS (pH 7.4) at 37°C for 1 hr. Such as image 3 As shown, the LC-MS / MS results show that ethynylsulfonate 2f can undergo efficient click reaction with azide-modified proteins.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com