A kind of protein labeling method of ethynyl sulfate click reaction
A click reaction and protein labeling technology, applied in the fields of chemical biology and biology, can solve the problem that probes are difficult to be absorbed by cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Example 1: Synthesis of ethynyl sulfur salts and efficient click reactions with azides to generate 1,5-triazole cycloaddition products.
[0029] Based on previous literature, we synthesized a series of ethynyl sulfate substrates by a one-pot method. Synthesis of Ethynyl Sulfide from Dimethyl Sulfoxide The reaction equation is as follows:
[0030]
[0031] In a 100-mL three-necked flask, sulfoxide (5 mmol, 1.0 equiv.) was dissolved in 40 mL of dichloromethane with nitrogen ventilation, cooled to -50°C, and trifluoromethanesulfonic anhydride (5 mmol, 1.0 equiv.) was added dropwise. equiv.) was stirred at this temperature for 1 hour, and trimethylsilylalkynyl (5 mmol, 1 equiv.) was dissolved in 5 mL of dichloromethane and added dropwise to the reaction solution. The temperature was slowly raised to -15° C. and stirred for 6 hours. After the reaction was completed, the solvent was revolved to dryness in vacuo, and the crude product was recrystallized to obtain product...
example 2
[0041] Example 2: X-ray diffraction analysis of 1,5-triazole cycloaddition single crystal product 3g.
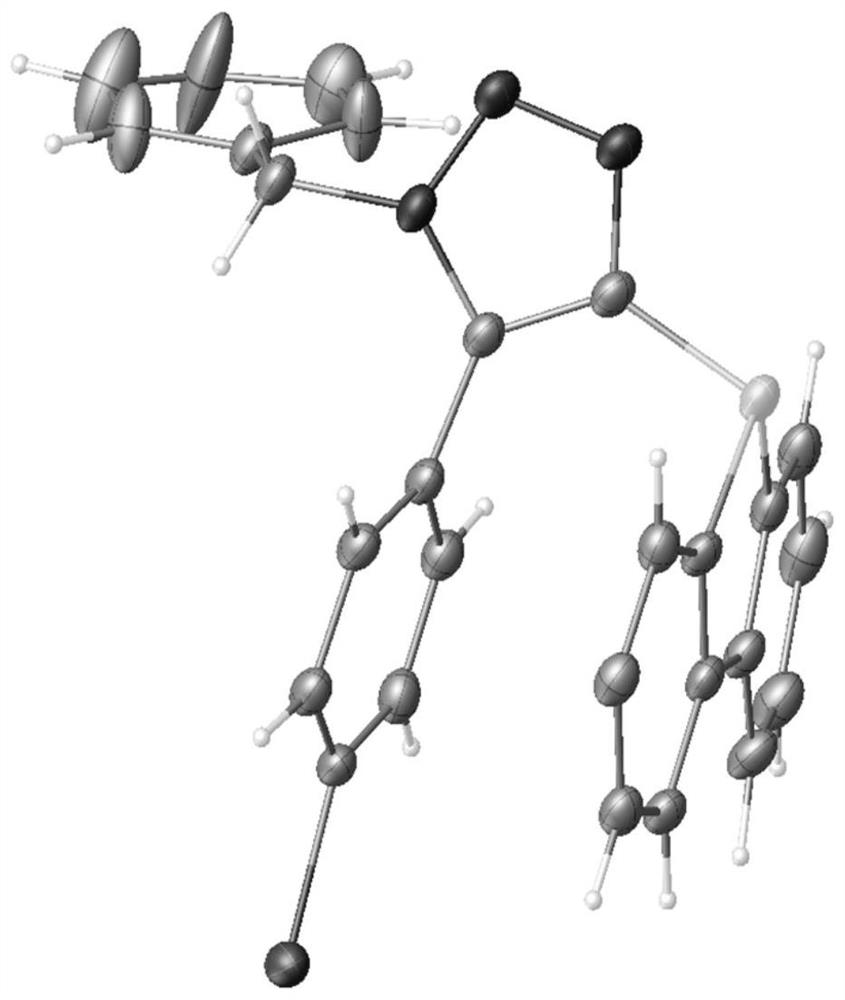
[0042] In order to fully elucidate the product structure, we carried out careful nuclear magnetic resonance (NMR) experiments and X-ray diffraction analysis of the 3g single crystal of the product 3g prepared by the method of Example 1, as shown in figure 2 shown. The distances of the C-N, C-C and N-N bonds in the cycloaddition structure of 1,5-triazole are consistent with the results for the five-membered ring.
example 3
[0043] Example 3: Secondary Mass Spectrometry Identification of the Use of Ethynyl Sulfate for Protein Labeling.
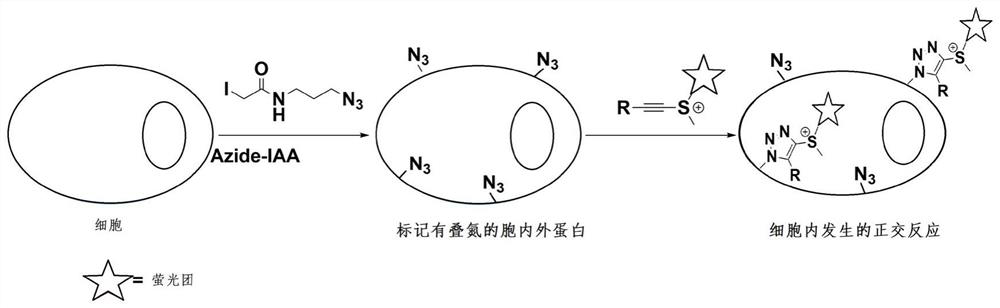
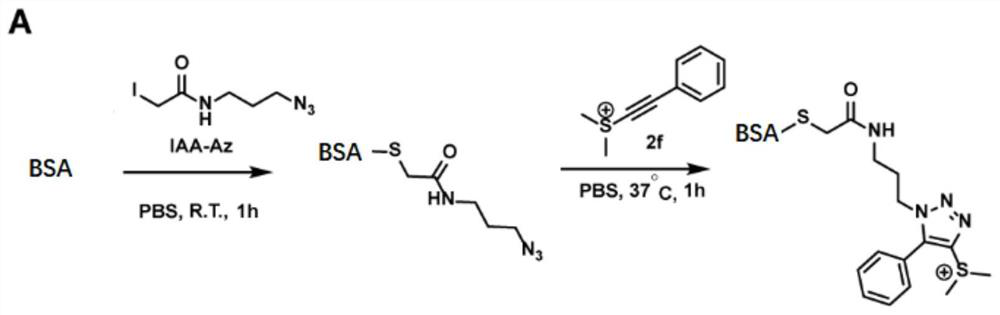
[0044] After confirming that ethynyl sulfur salts readily click-react with azides, we performed protein labeling experiments. In this labeling reaction, 2.5 mg / mL bovine albumin (BSA) was incubated with 1 mM azide alkylating reagent IAA-Az for 1 h at room temperature to install a bioorthogonal N 3 group, and then only incubated with ethynyl sulfate 2f in phosphate buffered saline PBS (pH 7.4) at 37°C for 1 hour. like image 3 As shown, the LC-MS / MS results showed that ethynyl sulfate 2f could undergo an efficient click reaction with azide-modified proteins.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com