A kind of prasugrel hydrochloride compound
A technology for prasugrel hydrochloride and a composition, which is applied in the field of medical research and can solve the problems of increased bleeding risk and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
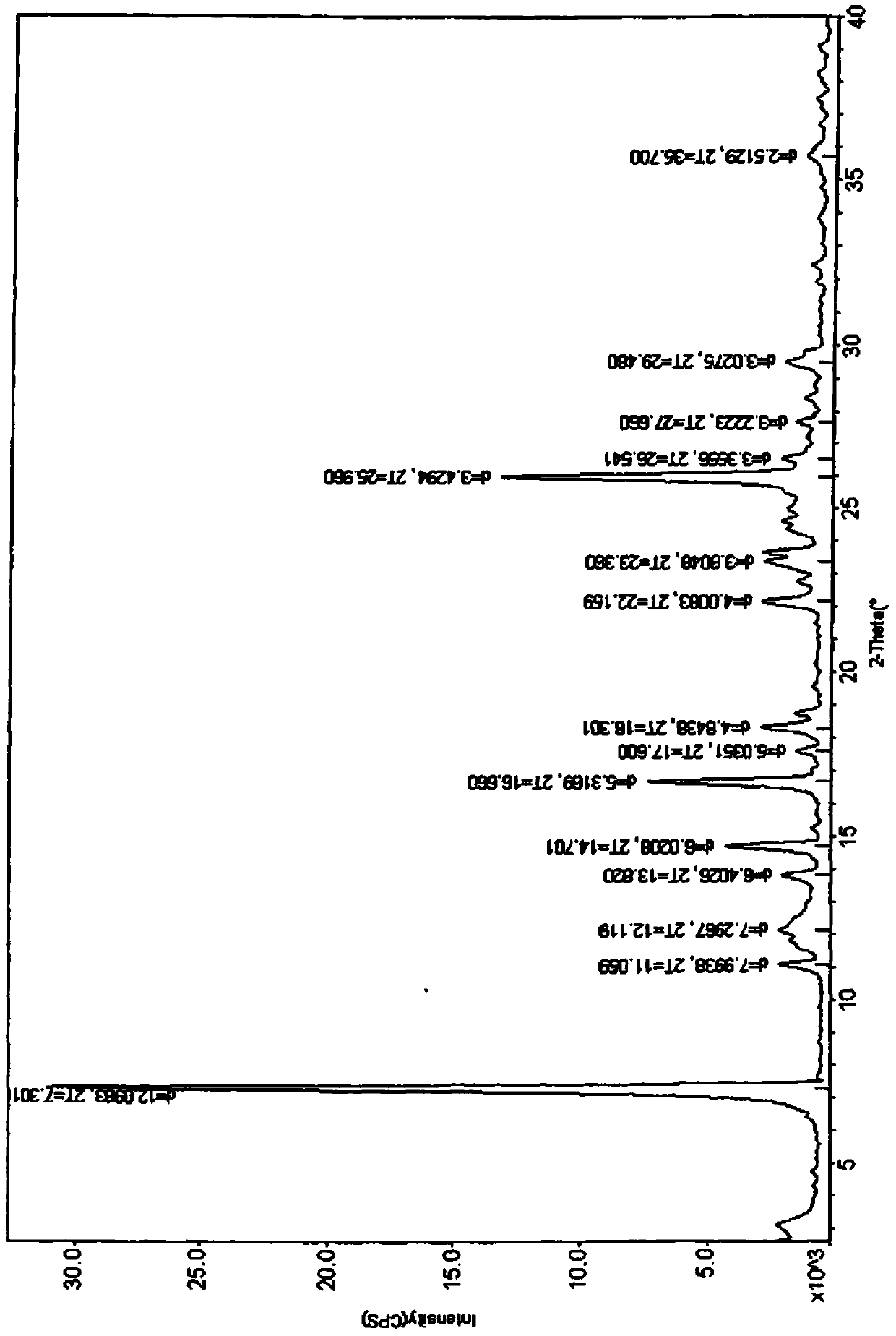
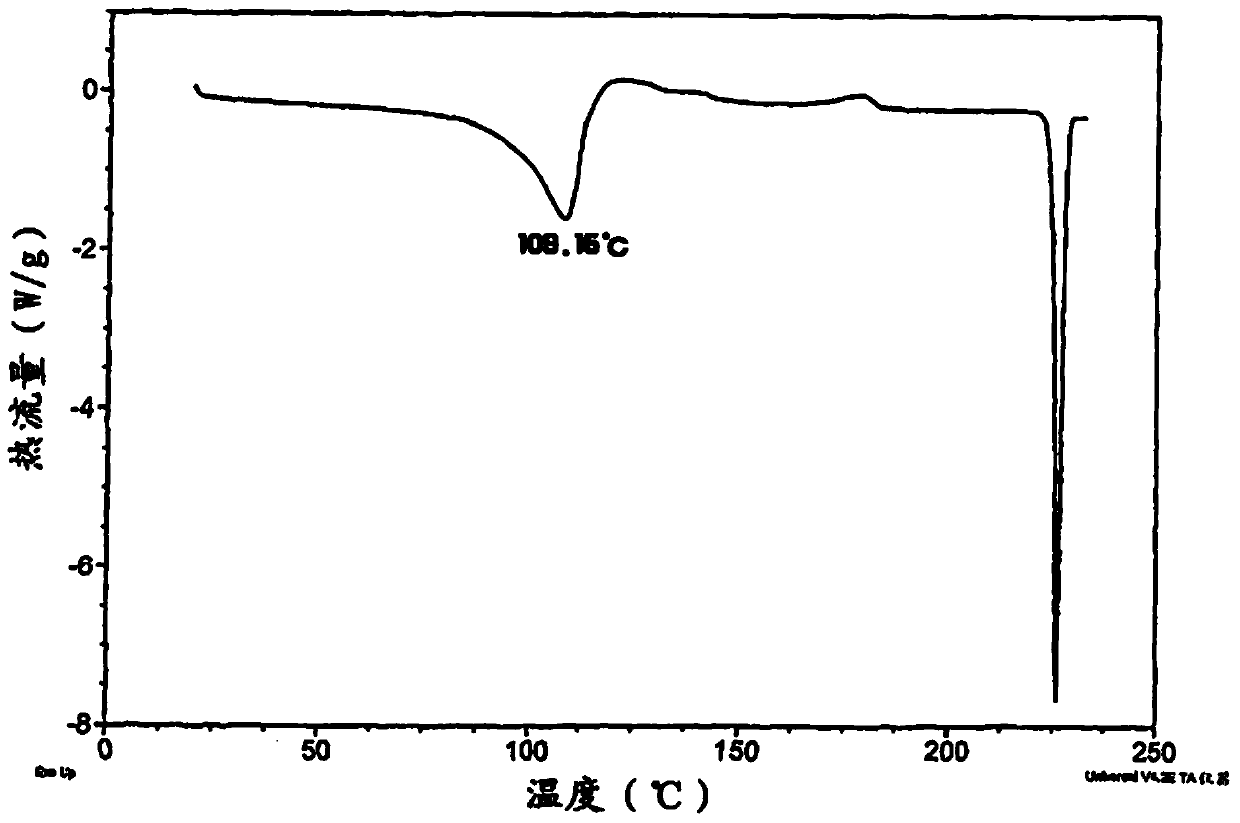
Image
Examples
Embodiment 1
[0020] Example 1 - Preparation of Prasugrel Hydrochloride
[0021] 2.2 kg of prasugrel free base and 7.7 liters of ethyl acetate were added to the reaction kettle, heated to about 50° C. with stirring, and the solution was clarified. Stop heating, slowly stir and cool down to 25±5°C, keep stirring for 1 h, and filter with suction. The filter cake was slurried with ether / ethanol (0.5 liter / 1 liter), rinsed with ethanol for 2L×2 times, and drained, and the wet weight was about 1.6 kg. After vacuum drying at 45±5℃ for more than 12 hours, about 1.3 kg of prasugrel free base fine products are obtained.
[0022] Take another reactor, add 1.20 kilograms of the above-obtained prasugrel free base fine products and 12 liters of acetone (weight ratio) containing 10% β-pinene, and heat up to about 30° C. to stir and dissolve. Suction filtration, the filter paper is rinsed with 2L of acetone (weight ratio) containing 10% β-pinene, the filtrates are combined and poured into a clean reacti...
experiment example 1
[0025] Experimental Example 1 - Raw Material Metabolism and Efficacy
[0026] Experimental animals: 32 male Wistar rats, weighing 200-230 g, were purchased from Sichuan University.
[0027] Experimental method: Rats were randomly divided into 4 groups, with 8 rats in each group. They were reared under normal conditions and had free access to water. After 12 hours of fasting, each group was given Prasugrel hydrochloride (Example 1) with a solid gavage device, respectively, at a dose of 25 , 50, 100 and 150 mg kg -1 . Blood collection time points: 300 blood samples were collected from the retroocular venous plexus before administration and at 5, 15, 30 minutes and 1, 2, 3, 4, 6, 8, 10, 12, 24, 36, and 48 hours after administration. -500 microliters in heparinized Ep tubes.
[0028] Sample processing method: Heparin anticoagulated venous blood was centrifuged at 5000 r / min for 10 minutes at room temperature, 100 μl of plasma was taken, 1300 μl of ethyl acetate was added, vorte...
experiment example 2
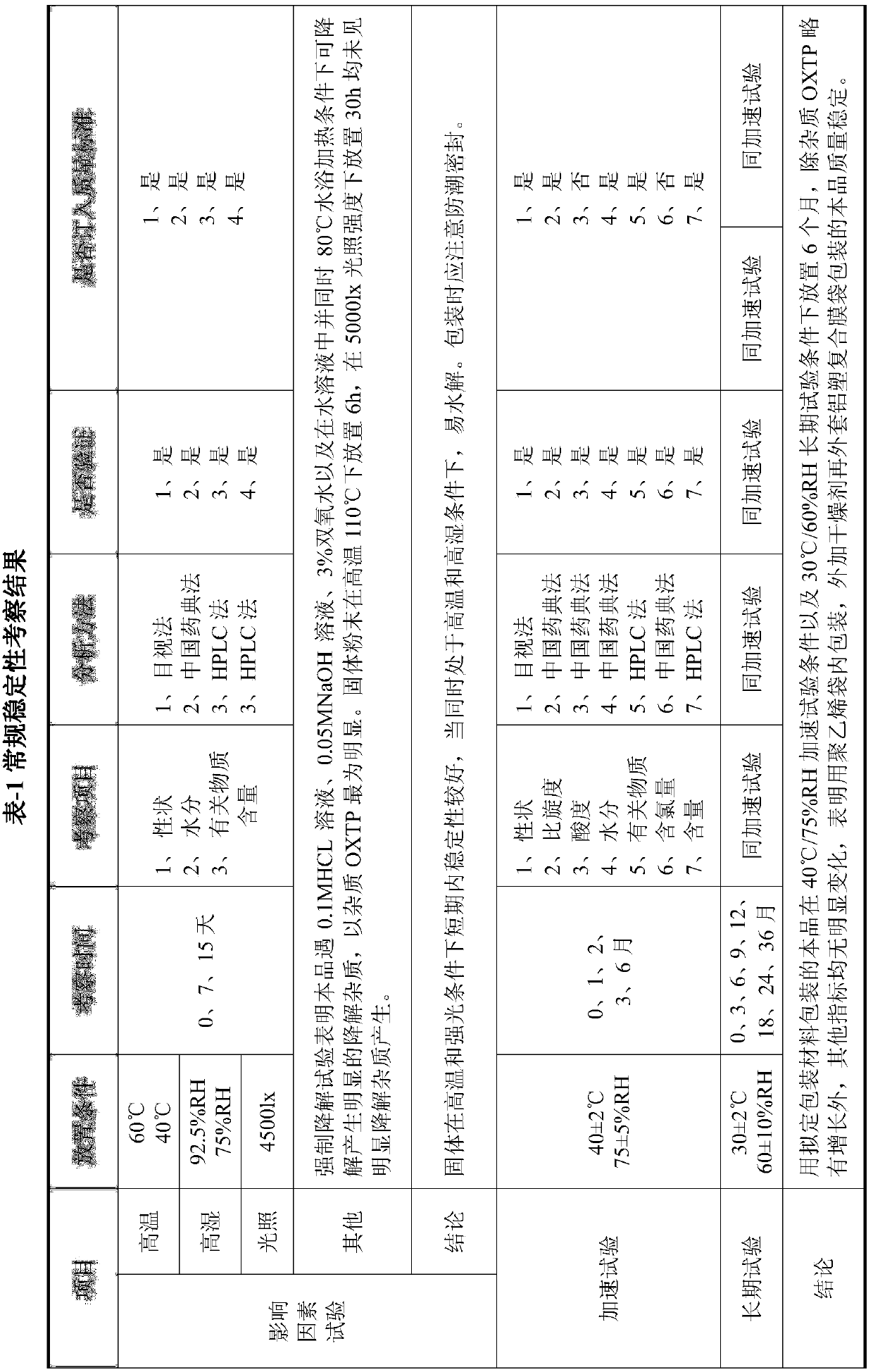
[0030] Experimental Example 2 - Stability Investigation of Raw Materials
[0031] The present invention carried out the 6-month accelerated test (40°C±2°C, 75%±5%RH) and the 6-month long-term test (30°C±2°C, 65%±5%RH) of Example 1. For Example 1, the long-term test stability investigation will continue, and the investigation indicators include properties, specific rotation, acid value, moisture, related substances, chlorine content and content.
[0032]
[0033]
[0034] .accelerated test
[0035] Packing: packed in medicinal low-density polyethylene bag, and then covered with aluminum-plastic composite film bag after adding desiccant
[0036] Inspection conditions: 40℃±2℃, 75%±5%RH
[0037] Table-3 Prasugrel hydrochloride accelerated test results (40℃±2℃, 75%±5%RH)
[0038]
[0039] .Long term test
[0040] Table-4 Prasugrel hydrochloride long-term test results (30℃±2℃, 60%±5%RH)
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com