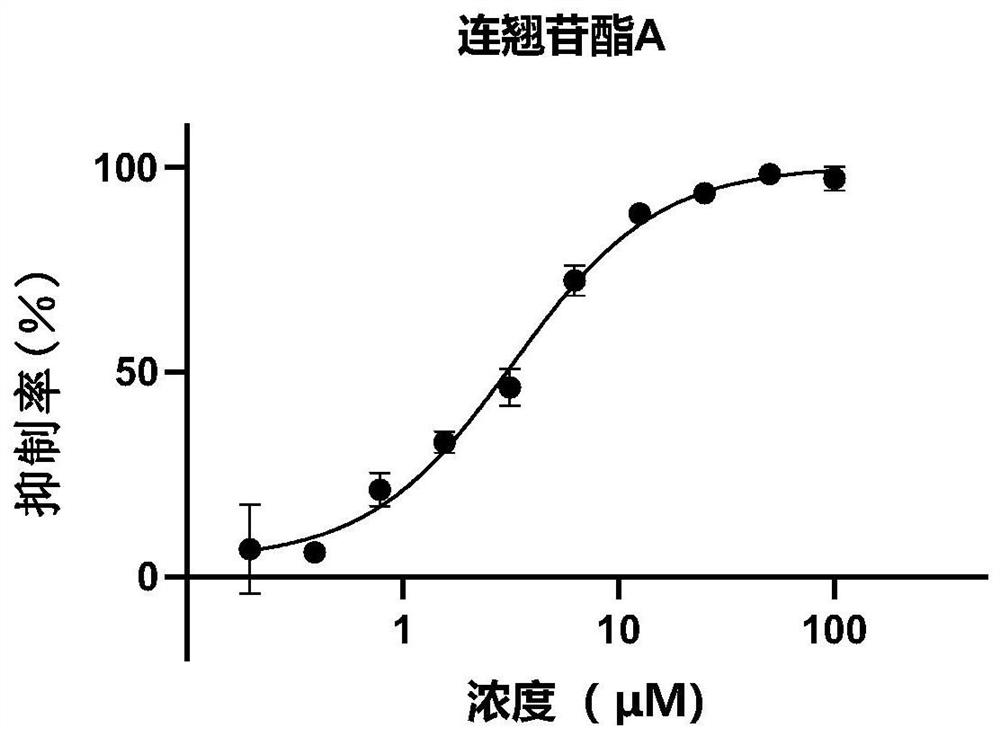
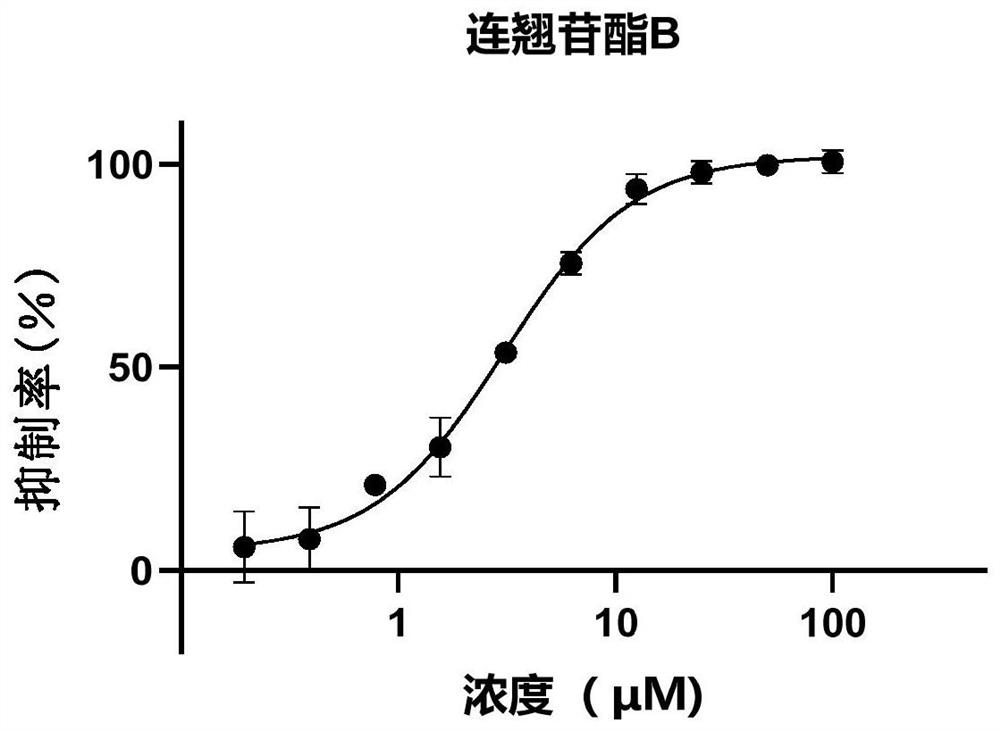
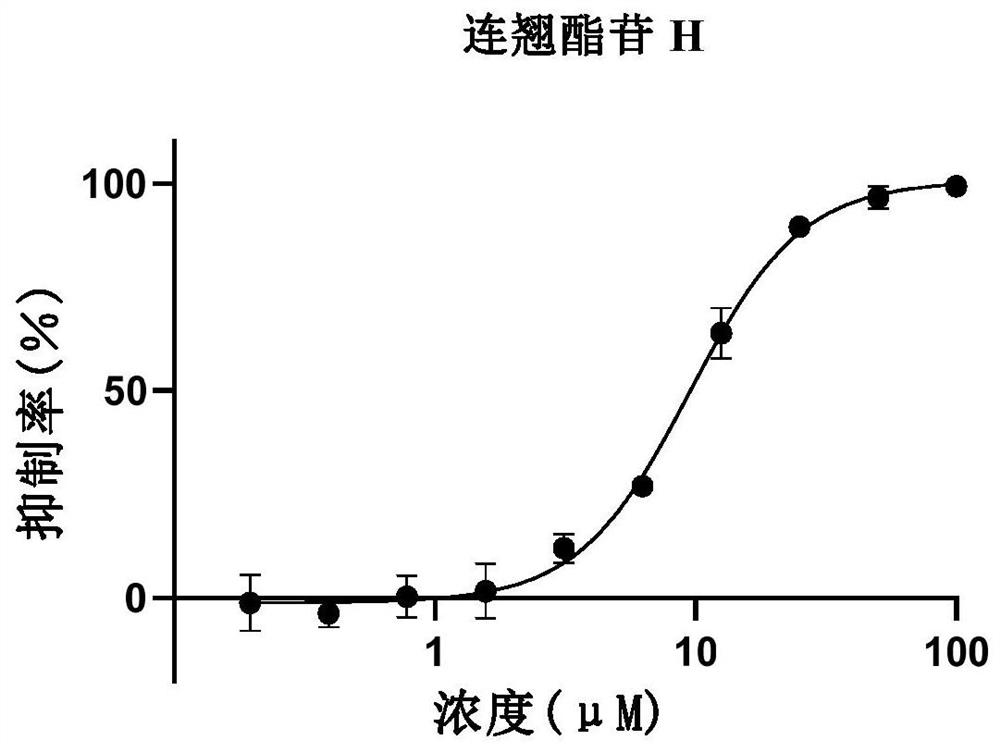
Application of phenylethanoid glycoside compound and composition thereof in preparation of medicine for preventing and treating new coronal pneumonia
A composition and drug technology, applied in the field of medicine, can solve the problems of vaccines and antiviral drugs without specific effects for severe pneumonia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Example 1: Isolation and structural identification of phenylethanol glycosides in Forsythia
[0157] 1.1. Method
[0158] Forsythia fruit (5Kg) purchased from Shanghai Tongrentang was crushed and soaked in 70% industrial ethanol (50L) at room temperature for 3 times, each time for 7 days. After the ethanol extracts were combined and concentrated, the obtained fluid extract (about 1Kg) was suspended in 15L of distilled water and then extracted 3 times with ethyl acetate (10L) to remove the middle and low polar components; the water layer was concentrated to 3L and then applied to a macroporous resin column Chromatography (AB-8, 3Kg) was sequentially eluted with 25 L of water, 10% ethanol, 40% ethanol, 70% ethanol and 95% ethanol. According to HPLC-MS analysis, forsythiaside components were mainly concentrated in the elution site of 40% ethanol. 40% of the eluate was concentrated and the obtained extract was taken 10 g, mixed with OSD, and then subjected to ODS (RP-C18,...
Embodiment 2
[0168] Example 2: Inhibitory effect of forsythiaside compounds on 2019-nCoV-3CLpro
[0169]The inhibitory activity of phenylethanol glycosides in Forsythia glycosides on 2019-nCoV3CLpro enzyme activity was evaluated by fluorescence resonance energy transfer method. The volume of the entire enzymatic reaction system is 120 μL, the final concentration of protease is 30 nM, and the final concentration of substrate is 20 μM. The buffer of the reaction system includes 50mM Tris pH7.3, 1mM EDTA. Add samples such as 2019-nCoV 3CLpro protease and different concentrations of compounds or mixtures to the 96-well plate, incubate at 30°C for 10 minutes, add the substrate and quickly put it into a microplate reader for reading. Excitation light and emission light were 340 nM and 405 nM, respectively. The test time is 10min, and the fluorescence value is read every 30s. In the final result, the reading value of the first 2 minutes was used to fit the reaction rate, and compared with the ...
Embodiment 3
[0178] Example 3: Isolation and structural identification of other phenylethanol glycosides
[0179] With reference to the literature and the above-mentioned preparation method, salidroside (Salidroside), pyloroside A1 (Jionoside A1) and B1 (Jionoside B1), isergoside (Isoacteoside), echinacoside (Echinacoside) and cistanche glycoside A (Cistanoside A), Poliumoside, Angoroside C (Angoroside C), Calceolarioside B (calceolarioside B), Desrhamnosylmartynoside (Desrhamnosylmartynoside) were obtained from Rhodiola rosea (Rhodiola rosea), Radix Rehmanniae Praeparata, Cistanche tubulosa, Callicarpa kwangtungensis, Scrophularia ningpoensis, Akebia quinata, Scutellaria prostrata. The structures of the compounds were identified by 1H NMR, 13C NMR and mass spectrometry.
[0180] Salidroside: white powder, ESI-MS m / z 299.3[M-H]+; 1H NMR (400 MHz, MeOH-d4) phenylethyl alcohol fragment: δ7.06 (2H, d, J = 8.6Hz, H-2,6),6.69(2H,d, J=8.6Hz,H-3,5),2.83(2H,m,H-7),3.70 and 4.03(each 1H,m,H-8a,8b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com