Polypeptide for preventing or treating ulcerative colitis
A technology for ulcerative colitis and its use, applied in the field of peptides used for the prevention or treatment of ulcerative colitis, can solve the problems of patients' physical and mental health and quality of life, difficult medication, poor efficacy, etc. Injury, reduce spleen weight index, inhibit the effect of monolayer cell permeability increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The separation, purification and determination of embodiment 1 polypeptide
[0058] Carry out probiotic fermentation to apples and wheat germ according to the following process conditions: crush 200 grams of apples and 200 grams of wheat germ with a juice extractor for 5 minutes, squeeze the juice and dissolve the residue in 4L of water. The mixture was heated at 70° C. for 1 h, extracted with ultrasound for 30 min, and centrifuged at 8450×g for 10 min to obtain raw wheat germ apple juice. Using 6% (v / v) mixed lactic acid bacteria (Lactobacillus bulgaricus: Lactobacillus casei: Lactobacillus plantarum: Lactobacillus helveticus=1:1:1:1, OD600=1.9) to inoculate the raw wheat germ apple juice after pasteurization, The fresh wheat germ apple fermented juice was obtained by static culture at 37°C for 24 hours.
[0059] The above fresh wheat germ apple fermented juice was dissolved in 75% ethanol for 30 minutes, and centrifuged at a speed of 8450×g for 10 minutes. The centr...
Embodiment 2
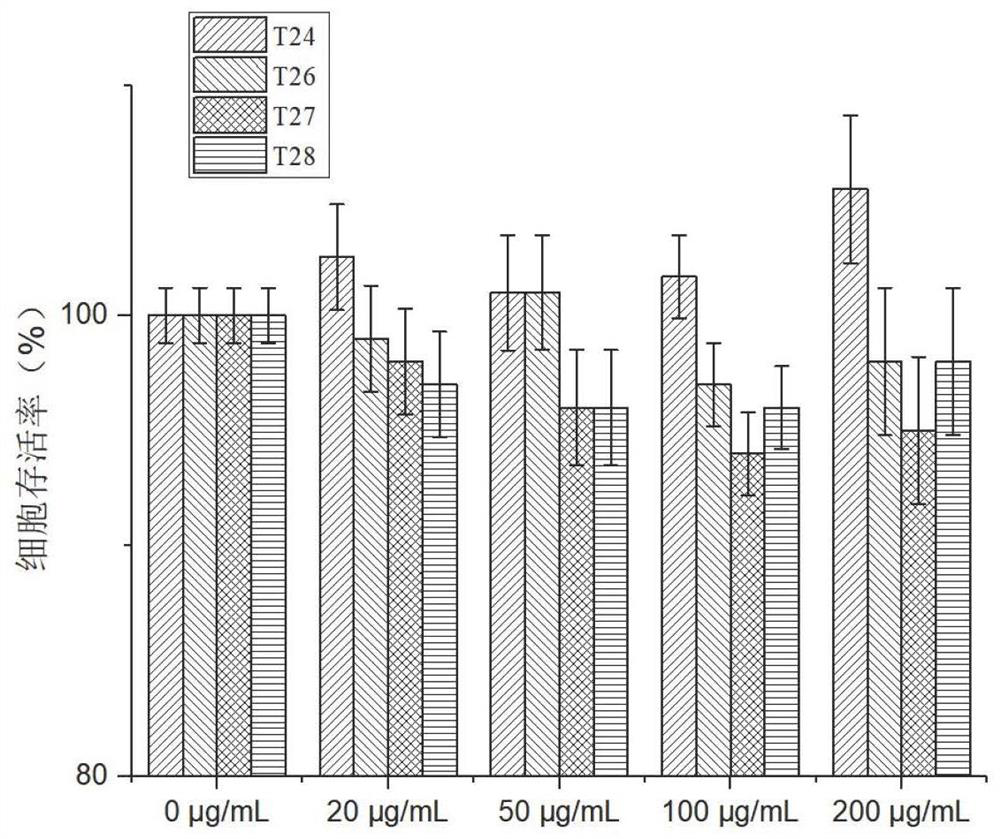
[0063] Example 2 Effects of Polypeptides on NCM460 Intestinal Epithelial Cells
[0064] The cytotoxicity of NCM460 cells treated with different concentrations of polypeptides for 24 hours was detected by CCK-8 method.
[0065] Among them, CCK-8 solution was purchased from Beijing Suo Laibao Biotechnology Co., Ltd., and NCM460 cells were purchased from Shanghai Guandao Bioengineering Co., Ltd. The specific assay method is as follows: inoculate cell suspension (400 μ L / well) in 24 well plates, and the number of cells is 10 4 / well, and add a final volume of 100ug / ml polypeptide, put the culture plate in the incubator for 12h pre-cultivation (37 ℃, 5% CO 2 ). Add 40 μL of CCK-8 solution to each well. The plates were incubated in the incubator for 2 hours. The absorbance at 450 nm was measured with a microplate reader. Vitality calculation refers to the following formula: cell viability (%) = [A (dosing) - A (blank)] / [A (0 dosing) - A (blank)] × 100; where, A (dosing): has ...
Embodiment 3
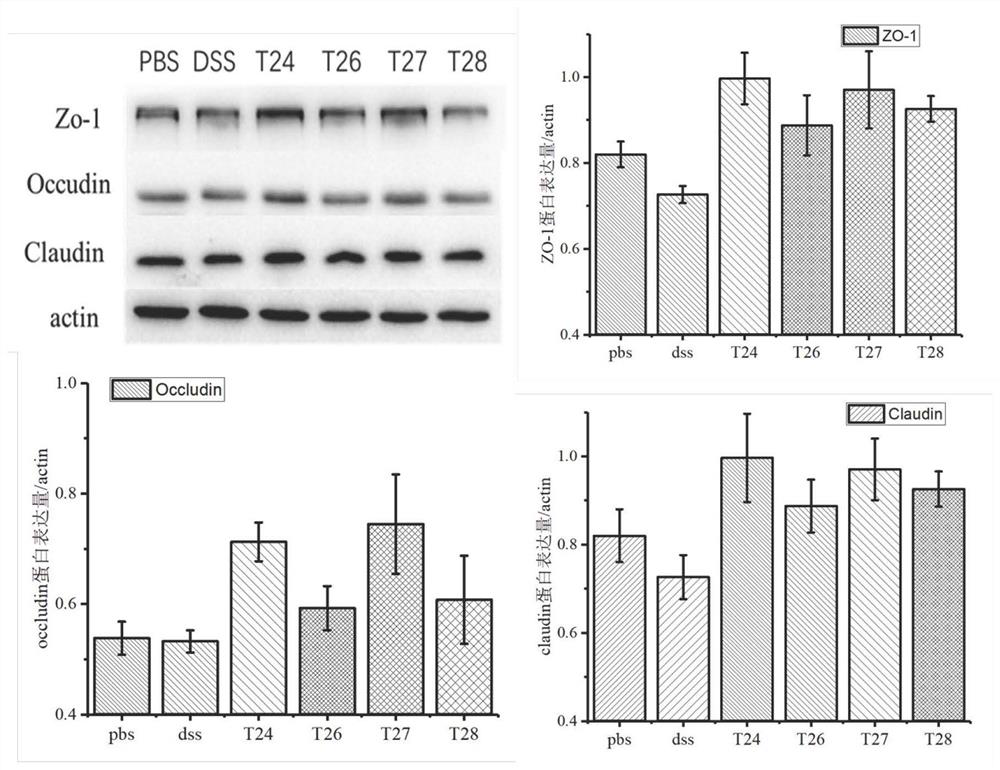
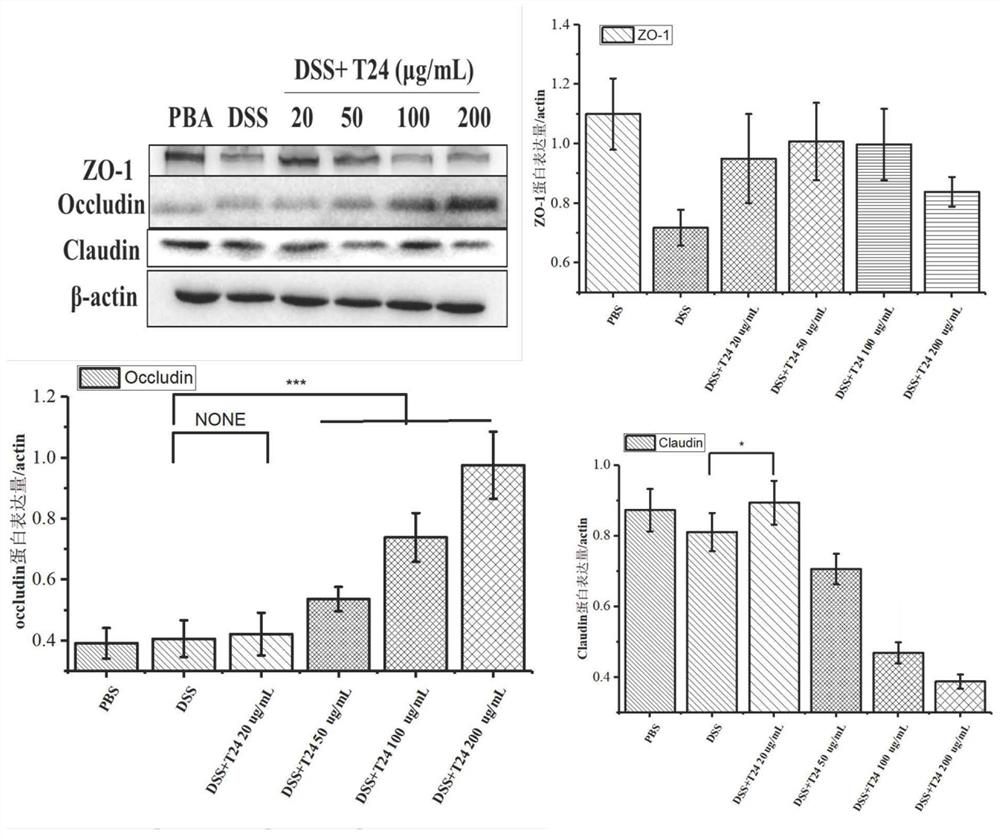
[0068] Example 3 Effects of Polypeptides on the Expression of Tight Junction Proteins
[0069] In order to study the effects of polypeptides T24, T26, T27 and T28 on the expression of tight junction proteins, the effects of adding DSS on the expression of ZO-1, claudin 1 and occludin1 were carried out by western blot. Wash NCM460 cells by lysing PBS twice with 1× protein loading buffer (50mm Tris-HCl-pH6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 12.5mm EDTA, 0.02% bromophenol blue) , Grind for 5 min with a grinding rod. The lysate was collected, heated at 100°C for 15 minutes, centrifuged at 13000×g for 5 minutes, and the supernatant was loaded into 15% SDS-PAGE for protein electrophoresis. The gel was transferred to a PVDF membrane (Millipore, MA, USA) at a constant flow of 300 mA for 2 hours. Blots were blocked with 5% (w / v) skim milk in PBST and incubated with 1:1000 diluted antibody for 2 hours. All primary antibodies were purchased from CST Biotechnology (MA, USA)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com