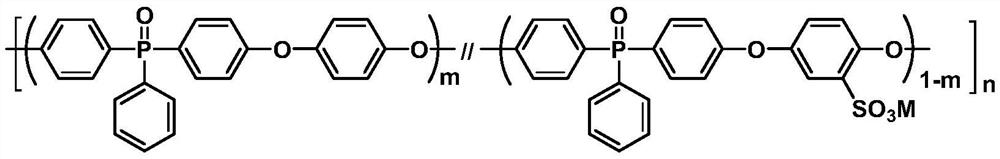
Sulfonated aromatic phosphine oxide polymer containing hydroquinone and preparation method and application thereof
A hydroquinone and sulfonated aromatic technology is applied in the field of sulfonated aromatic phosphine oxide polymer and its preparation, which can solve the problems of low hydrogen permeability and the like, and achieve the effects of simple purification, mild conditions, low price and easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
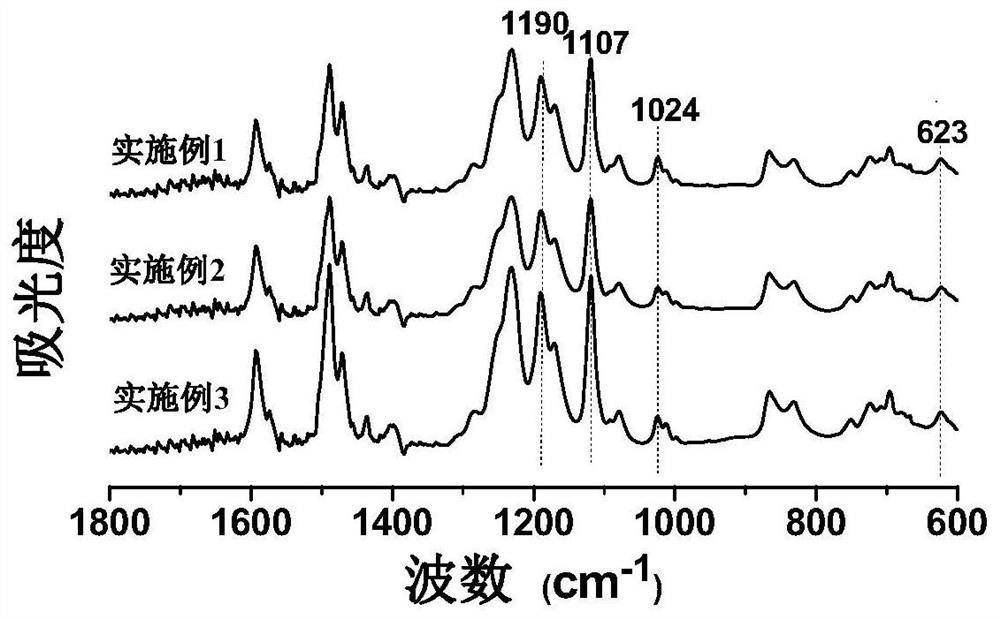
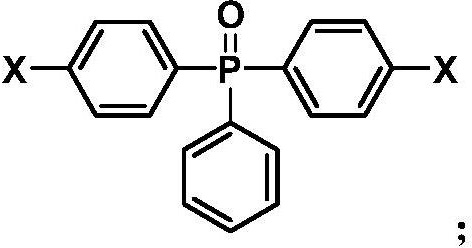
[0039] Add 4.0 mmol (1.2571 g) of bis(4-fluorophenyl) phenylphosphine oxide, 1.8 mmol (0.4109 g) of 2,5-di Potassium hydroxybenzenesulfonate, 2.2 mmol (0.2422 g) of hydroquinone and 4.50 mmol (0.6218 g) of K 2 CO 3 , and then add 8 mL of N-methyl-2-pyrrolidone and 8 mL of toluene into the flask, pass through nitrogen protection, and stir evenly at room temperature. Raise the temperature to 150°C for reaction, maintain reflux for 4 hours, discharge toluene, raise the temperature and react at 175°C for 24 hours, then continue to raise the temperature to 190°C until the reaction solution is very viscous. Lower the reaction temperature to 120°C, add 2 mL of N-methyl-2-pyrrolidone into the three-necked flask to dilute the reaction solution, then pour it into 300 mL of deionized water, and stir while pouring to obtain brown strips. Soak the product in hot water to remove inorganic salts, dry it in vacuum for 24 hours, and set it aside.
[0040] Implementation effect: yield 92%, n...
Embodiment 2
[0042]Add 4.0 mmol (1.2571 g) bis(4-fluorophenyl) phenylphosphine oxide, 2.0 mmol (0.4563 g) 2,5-bis Potassium hydroxybenzenesulfonate, 2.0 mmol (0.2202 g) of hydroquinone and 4.55 mmol (0.6294 g) of K 2 CO 3 , and then add 8mL N-methyl-2-pyrrolidone and 8mL toluene into the flask, pass through nitrogen protection, and stir evenly at room temperature. Raise the temperature to 150°C for reaction, maintain reflux for 4 hours, discharge toluene, and continue to react at 175°C until the reaction solution is very viscous. Lower the reaction temperature to 120°C, add 2 mL of N-methyl-2-pyrrolidone into the three-necked flask to dilute the reaction solution, then pour it into 300 mL of deionized water, and stir while pouring to obtain brown strips. Soak the product in hot water to remove inorganic salts, dry it in vacuum for 24 hours, and set it aside.
[0043] Implementation effect: yield 92%, number average molecular weight M n =3.17×10 4 , weight average molecular weight M w...
Embodiment 3
[0045] Add 4.0 mmol (1.2571 g) bis(4-fluorophenyl) phenylphosphine oxide, 2.2 mmol (0.5022 g) 2,5-bis Potassium hydroxybenzenesulfonate, 1.8 mmol (0.1982 g) of hydroquinone and 4.61 mmol (0.6370 g) of K 2 CO 3 , and then add 8 mL of N-methyl-2-pyrrolidone and 8 mL of toluene into the flask, pass through nitrogen protection, and stir evenly at room temperature. Raise the temperature to 150°C for reaction, maintain reflux for 4 hours, discharge toluene, and continue to react at 175°C until the reaction solution is very viscous. Lower the reaction temperature to 120°C, add 2 mL of N-methyl-2-pyrrolidone into the three-necked flask to dilute the reaction solution, then pour it into 300 mL of deionized water, and stir while pouring to obtain brown strips. Soak the product in hot water to remove inorganic salts, dry it in vacuum for 24 hours, and set it aside.
[0046] Implementation effect: the productive rate is 93%, the number average molecular weight M n =3.32×10 4 , weight...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com