Novel coronavirus trimer recombinant protein, DNA, mRNA, application and mRNA vaccine
A histone and weight technology, applied in the field of genetic engineering, can solve the problems of affecting immunogenicity, slow approval for marketing, insufficient exposure of antigenic epitopes, etc., to achieve high-efficiency expression increase, prevent structural rearrangement, and strong neutralizing antibodies effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention also provides a method for preparing the above-mentioned mRNA. The above-mentioned DNA is transcribed in vitro to obtain the mRNA. The nucleotide sequence of the mRNA in the present invention includes any one of the nucleotide sequences in (a) and any one of the nucleotide sequences in (b); wherein
[0042] (a): The nucleotide sequence encoding the amino acid sequence SEQ ID No.1 is preferably one of SEQ ID No.13~SEQ ID No.15; the nucleotide sequence encoding the amino acid sequence SEQ ID No.2 is preferably SEQ One of ID No.16~SEQ IDNo.18; the nucleotide sequence encoding amino acid sequence SEQ ID No.3 is preferably one of SEQ ID No.19~SEQ IDNo.21; encoding amino acid sequence SEQ ID No The nucleotide sequence of .4 is preferably one of SEQ ID No.22~SEQ IDNo.24; the nucleotide sequence of encoding amino acid sequence SEQ ID No.5 is preferably among SEQ ID No.25~SEQ IDNo.27 kind of. (b): The nucleotide sequence encoding the amino acid sequence S...
Embodiment 1
[0055] Embodiment 1 mRNA vaccine preparation method
[0056] 1. The constructed expression plasmid is amplified as the DNA template according to the following reaction system:
[0057] Reaction volume, 50μl (reaction volume of a single tube, multiple tubes can be reacted at the same time)
[0058] Premix (2×) PCR amplification system (50 μl): PrimeSTAR Max 25 μl, Substance F (SEQ ID No.5, 10 μmol / L) 1.2 μl, Primer R (SEQ ID No.5, 10 μmol / L) 1.2 μl , DNA template (1ng / μl) 1μl and water 21.6μl.
[0059] The PCR amplification program is as follows: pre-denaturation at 98°C for 3min; denaturation at 98°C for 10s, annealing at 60°C for 5s, extension at 72°C for 2min, 34 cycles; final extension at 72°C for 10min.
[0060] After the reaction, the reaction solution was combined into a 1.5ml Tube tube. Take 10 μl for DNA agarose gel electrophoresis (1.5% agarose, 5V / min, 40min). Confirm the success of the reaction according to the size of the electrophoresis target band.
[0061] ...
Embodiment 2
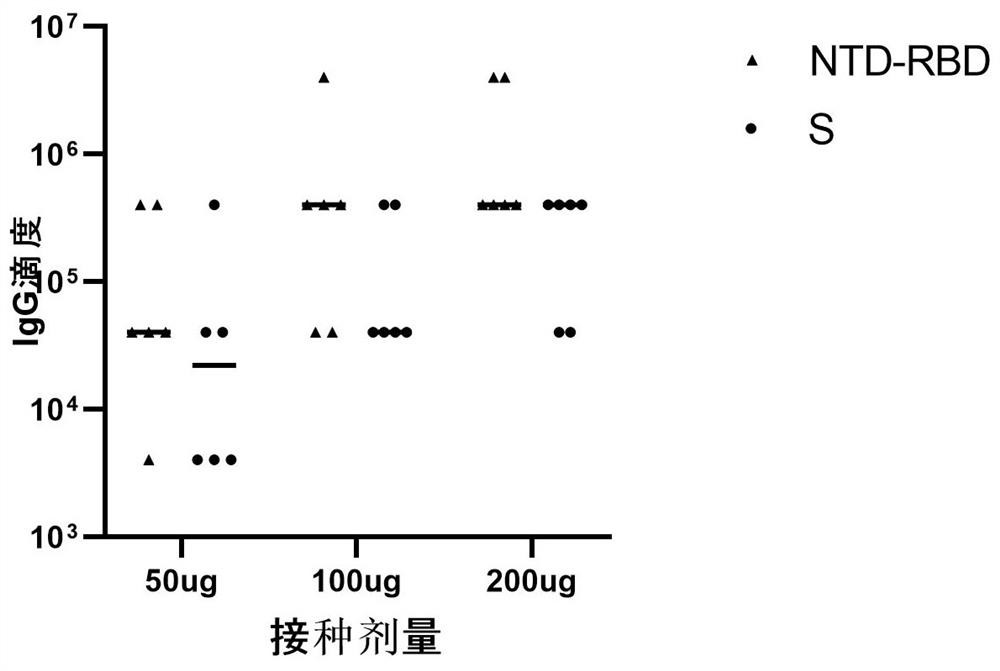
[0110] Example 2 Comparative test of full-length S protein mRNA vaccine and second-generation NTD-RBD mRNA vaccine
[0111] Select 6-week-old balb / c mice, and inoculate 50ug, 100ug, and 200ug of NTD-RBD mRNA vaccine and S protein full-length mRNA vaccine respectively, and inject twice on the 1st and 14th day, respectively, and take small mice on the 35th day. Rat serum was used to detect the titer of anti-S protein-specific antibody in the serum.
[0112] 1. Coating: Dilute S1 protein (Beijing Sino Biological Science and Technology Co., Ltd., 40591-MM43) with coating buffer to a solution of 200ng / ml, add it to the microtiter plate, the volume added to each well is 100μl, each dilution Repeat for 3 wells, cover with sealing film, and coat overnight at 4°C.
[0113] 2. Plate washing: Pour out the coating liquid on the coated 96-well plate, put it on the buckle absorbent paper, and buckle the plate hard until there is no residue in the well. Prepare the eluent, dilute 50× Washi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com