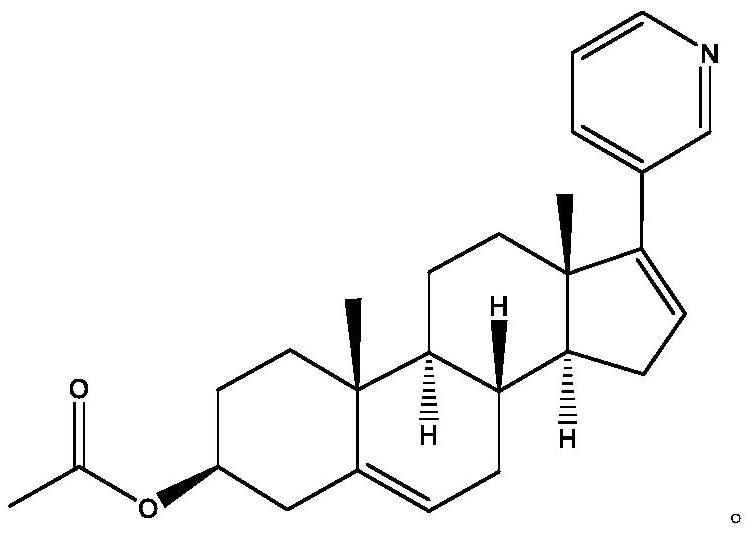
Abiraterone acetate suppository and preparation method thereof
A technology of abiraterone acetate and suppositories, which is applied in the field of medicine, can solve the problems of high bioavailability and failure to provide, and achieve the effects of simple preparation process, improved bioavailability, and small individual differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Component content
[0032] Aistelon 100g
[0033] Stealic acid polya carbide 1500g
[0034] Preparation Process:
[0035] The calculation is used to measure the stearate polyaptoalkoxide, heating melts, and adds the amount of acetate at the same amount of acetate, stirring, and dissolving, filled on the suppository filling line.
Embodiment 2
[0037] Component content
[0038] Aistelon 100g
[0039] Stealic acid polyaptoxate 1000g
[0040] Preparation Process:
[0041] The calculation is used to measure the stearate polyaptoalkoxide, heating melts, and adds the amount of acetate at the same amount of acetate, stirring, and dissolving, filled on the suppository filling line.
Embodiment 3
[0043] Component content
[0044] Aistelon 100g
[0045] Stealic acid polyluorodoxide 2000g
[0046] Preparation Process:
[0047] The calculation is used to measure the stearate polyaptoalkoxide, heating melts, and adds the amount of acetate at the same amount of acetate, stirring, and dissolving, filled on the suppository filling line.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com