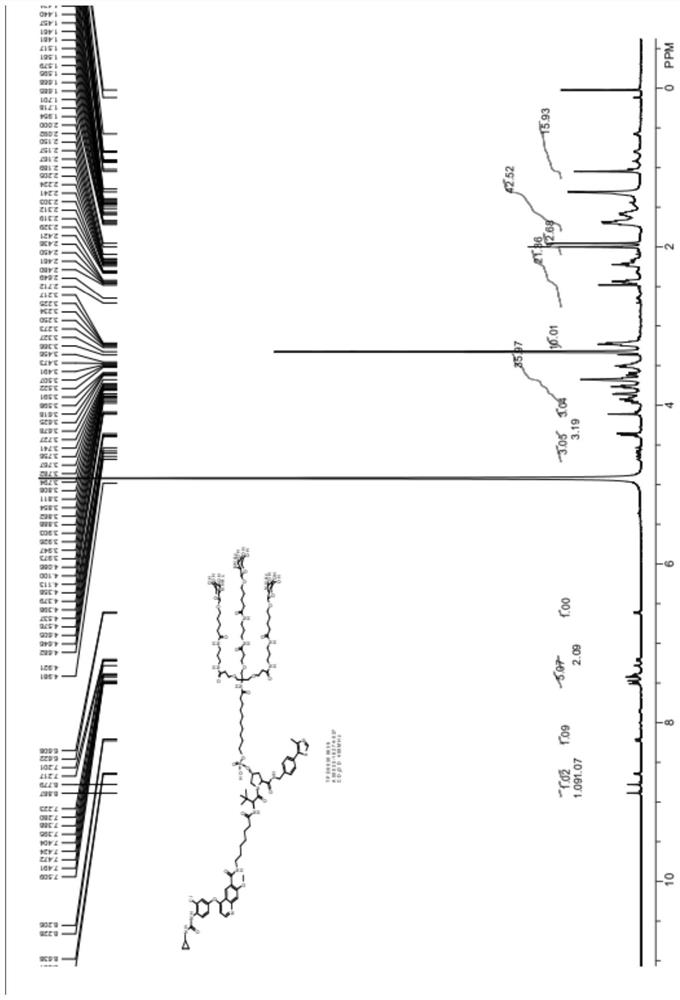
Tissue-targeted protein targeted-degradation compound and application thereof
A compound and solvate technology, applied in the field of pharmaceuticals, can solve problems that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Embodiment 1, the synthesis of compound WGint2
[0107]
[0108] A solution of 10-hydroxydecanoic acid (10.0 g, 53.2 mmol) in DMF (250 mL) was treated with potassium bicarbonate (5.32 g, 53.2 mmol) and benzyl bromide (9.10 g, 53.2 mmol), then at room temperature in Stir under argon for 18 hours. The solvent was evaporated and the residue was partitioned between EA and water. The aqueous layer was washed with EA, and the combined organic extracts were dried over anhydrous magnesium sulfate. The resulting crude product was purified by flash chromatography on silica gel to give compound WGint2 (8.0 g) as a white solid, LC / MS (ESI) m / z: [M+H] + 279.
Embodiment 2
[0109] Embodiment 2, the synthesis of compound WGint3
[0110]
[0111] To a solution of compound WGint2 (5.0 g, 18.0 mmol) in 100 mL of anhydrous dichloromethane containing tetrazolediisopropylamine (4.6 g, 27.0 mmol) was added 2-cyanoethoxy-N,N,N , N-tetraisopropylammonium diphosphate (9.0 mL, 27.0 mmol), and the mixture was stirred at room temperature for 6 h until TLC showed complete reaction. The dichloromethane was then removed by evaporation and the product was extracted in ethyl acetate, washed with 5% sodium bicarbonate solution and brine, dried over anhydrous sodium sulfate and evaporated to a small volume. The product was eluted on a silica gel column with 10-35% ethyl acetate-n-hexane+2% triethylamine to give compound WGint3 (2.5 g). This intermediate was directly used in the next reaction.
Embodiment 3
[0112] Embodiment 3, the synthesis of compound WGint6
[0113]
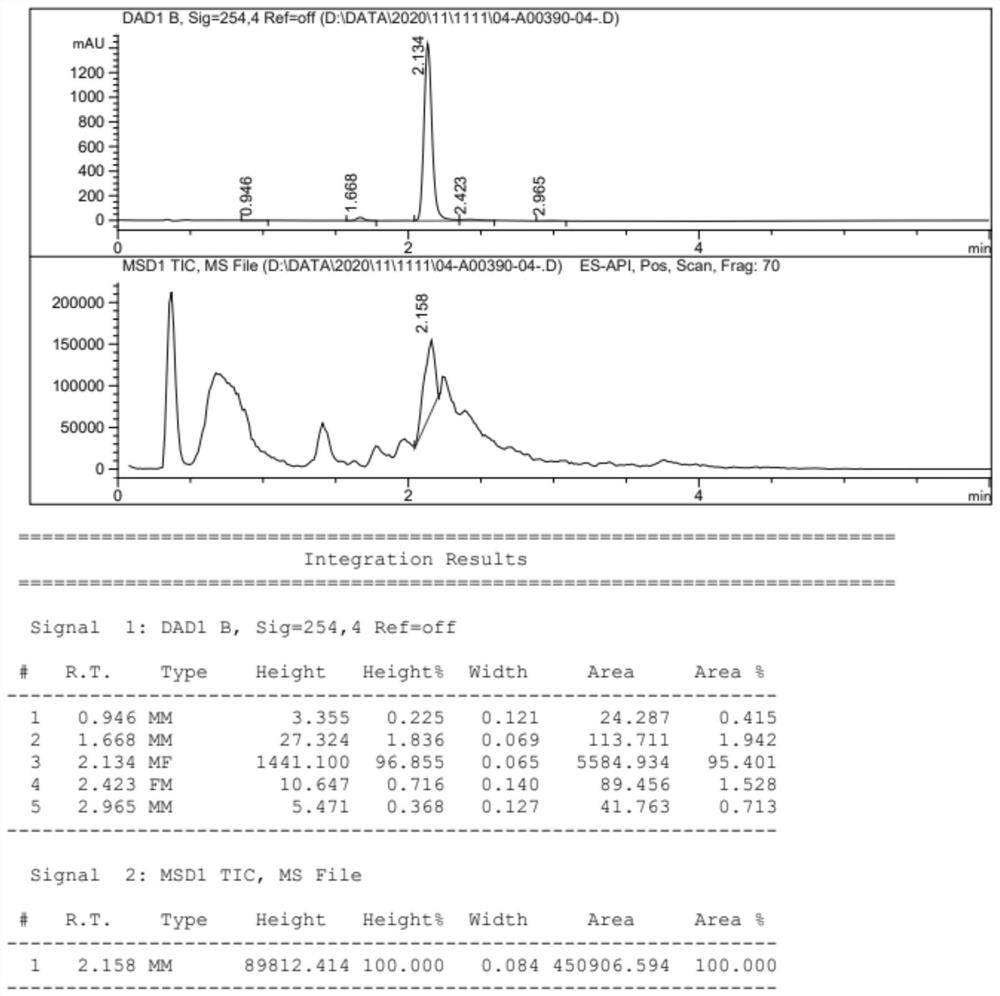
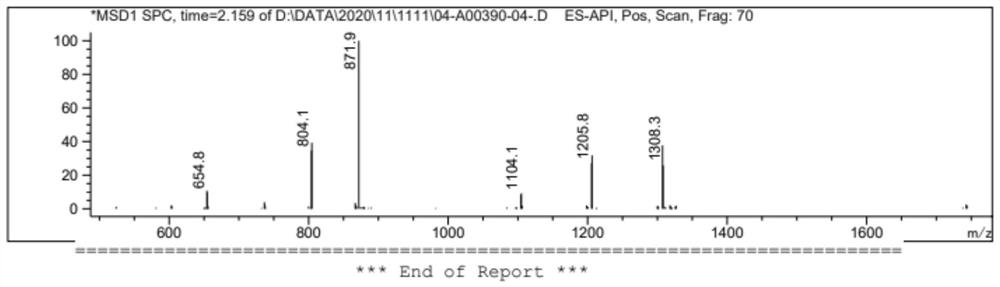
[0114] A mixture of compound WGint3 (2.5 g, 5.2 mmol) and compound WGint4 (0.5 g, 0.52 mmol) in ACN (25 mL) was added to tetrazole (145 mg, 2.1 mmol) and stirred overnight. tert-Butyl peroxide in decane (2 mL, 5M) was added dropwise at 0 °C, and the mixture was stirred at room temperature for 6 h. The reaction was monitored by thin layer chromatography. After completion of the reaction, the reaction mixture was concentrated at 40 °C, diluted with EA, washed with water and brine solution. The organic layer was dried over anhydrous Na2SO4, filtered and the solvent was evaporated to give crude compound WGint6. LC / MS(ESI)m / z:[M+H] + 1360.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com