Method for co-production of synthesis gas by carbonate hydrogenation refining for carbon dioxide emission reduction
A technology of carbon dioxide and carbonate, applied in the direction of carbon monoxide, calcium/strontium/barium oxide/hydroxide, silicate, etc., can solve problems such as ecological environment impact, increase production cost, and emit large carbon dioxide, and achieve reduction Decomposition temperature, improving economic benefits, and mitigating the effects of the greenhouse effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
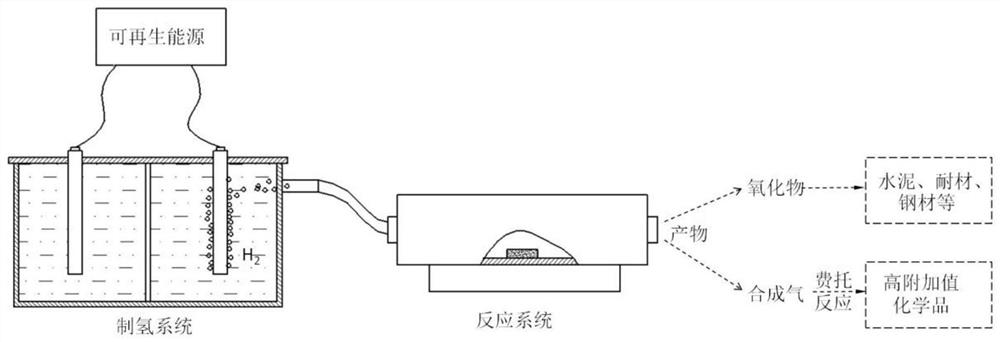
[0025] like figure 1 as shown,
[0026] Carry out the concrete steps of calcium carbonate hydrogenation refining calcium oxide co-production synthesis gas with the inventive method as follows:
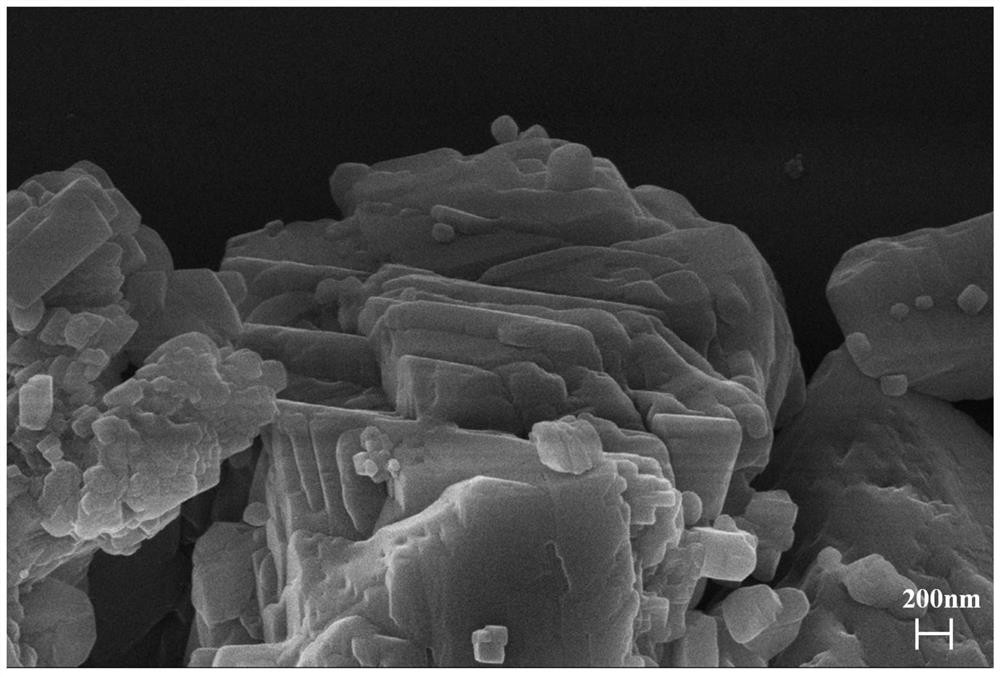
[0027] Using nickel-iron hydrotalcite as the anode, commercial Pt / C catalyst as the cathode, and an applied voltage of 2V, hydrogen is produced by electrolysis of water. The prepared gas is directly connected to the tube furnace after passing through the recovery system and the filtration system, and the gas flow rate of hydrogen is set to 100mL / min; 10g of calcium carbonate is placed in the tube furnace, and the pyrolysis temperature is set to 800°C, and the heating rate is The temperature is 10°C / min, and the reaction time is 60min. Calcium oxide product is obtained after roasting, and H in the syngas 2 The ratio with CO is 2:1. By adjusting the flow rate of hydrogen gas, the H in the resulting syngas can be controlled 2 The ratio range with CO is between (1:4) and (4:1).
[002...
Embodiment 2
[0030] Carry out the concrete steps of calcium carbonate / silicon dioxide hydrogenation refining calcium silicate co-production synthesis gas with the inventive method as follows:
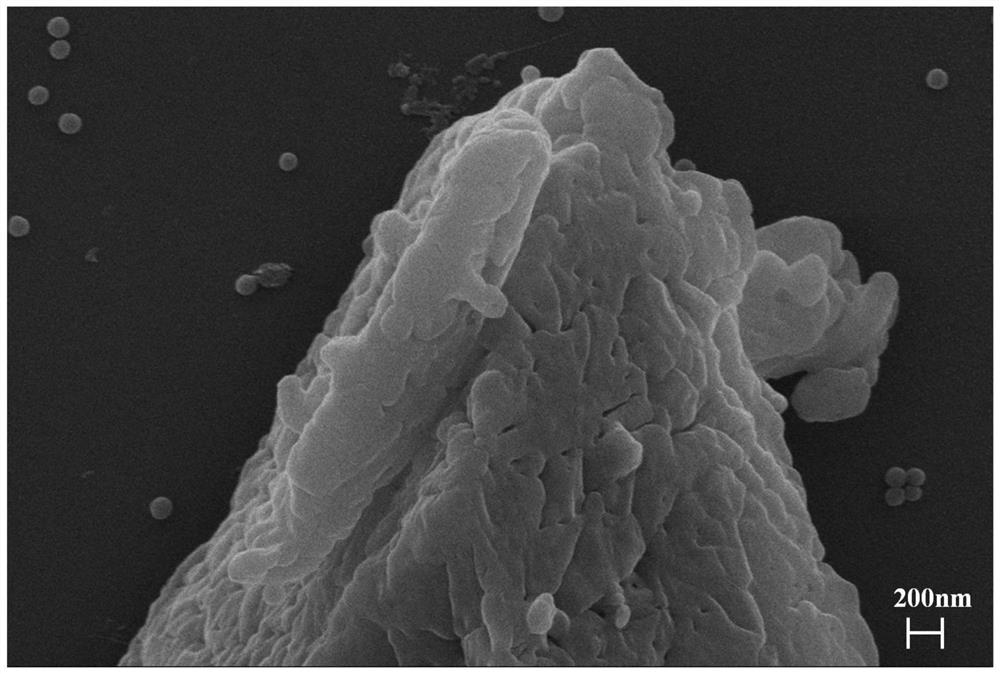
[0031] Using cobalt-iron hydrotalcite as the anode, commercial Pt / C catalyst as the cathode, and an applied voltage of 2V, hydrogen is produced by electrolysis of water. The prepared gas is directly connected to the tube furnace after passing through the recovery system and the filtration system, and the gas flow rate of hydrogen is set to 200mL / min; 50g of calcium carbonate / silicon dioxide mixed material (molar ratio is 1 / 1) is placed in the tube In the furnace, the pyrolysis temperature is set to 850°C, the heating rate is 5°C / min, and the reaction time is 200min. Calcium silicate product is obtained after roasting, and H in the synthesis gas 2 The ratio to CO is 1 / 1. By adjusting the flow rate of hydrogen gas, the H in the resulting syngas can be controlled 2 The ratio range with CO is between ...
Embodiment 3
[0034] Carry out the concrete steps of magnesium carbonate hydrogenation refining magnesium oxide co-production synthesis gas with the inventive method as follows:
[0035] With nickel-iron hydrotalcite as the anode and commercial Pt / C catalyst as the cathode, the light intensity was 100mW cm -2 , the applied voltage is 0.6V, and the production of hydrogen is realized through photoelectric water splitting technology. The prepared gas is directly connected to the tube furnace after passing through the recovery system and the filtration system, and the gas flow rate of hydrogen is set to 100mL / min; take 20g of magnesium carbonate and place it in the tube furnace, set the pyrolysis temperature to 900°C, and the heating rate The temperature is 20°C / min, the reaction time is 100min, the magnesium oxide product is obtained after roasting, and the H in the syngas 2 The ratio to CO is 1 / 1. By adjusting the flow rate of hydrogen gas, the H in the resulting syngas can be controlled 2...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap