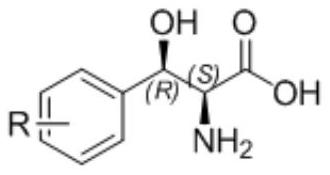
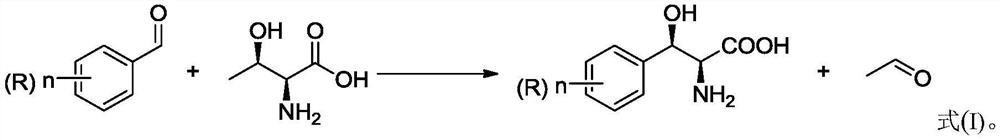
Modified threonine transaldolase and application thereof
A threonine and transaldolase technology, applied in the field of enzyme engineering, can solve the problems of low conversion rate, difficult product separation and purification, poor stereoselectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Example 1: Materials and methods
[0157] Unless otherwise specified, the experimental methods used in the present invention are all conventional methods, and details of gene cloning operations can be found in the aforementioned Sambrook et al., 1989.
[0158] i) Reagents and instruments:
[0159]DNA polymerase (PrimeSTAR Max DNAPolymerase) and DpnI endonuclease were purchased from TaKaRa Company;
[0160] The plasmid extraction kit was purchased from Axygen;
[0161] P-thymphenylbenzaldehyde was purchased from Aladdin, product number M185093, with a purity of 98%;
[0162] L-threonine was purchased from McLean, product number C10393311, analytically pure;
[0163] Pyridoxal phosphate was purchased from Aladdin, product number P136795, purity ≥ 98%;
[0164] Magnesium chloride was purchased from Aladdin, product number A2006034, analytically pure;
[0165] Oxidized nicotinamide adenine dinucleotide (NAD) was purchased from Aladdin, product number N196974, with a pu...
Embodiment 2
[0194] Embodiment 2, preparation and detection of the mutant of LTTA (LTTA01) of Pseudomonas fluorescens
[0195] Using the nucleic acid encoding LTTA01 (SEQ ID NO: 1) as a template, the mutant was prepared according to the method of Example 1. The obtained mutants are shown in Table 1. According to the method of Example 1, LTTA01 and its mutants were expressed by shaking flask culture, and enzyme powder was prepared. The activity of LTTA01 and its mutants was determined according to method 2 in item vi) of Example 1, and the results are shown in Table 1, wherein the relative activity refers to the activity of the mutant / the activity of LTTA01.
[0196] Table 1
[0197]
[0198]
Embodiment 3
[0199] Example 3: Preparation and detection of mutants of L-threonine transaldolase from different sources
[0200] According to the method of Example 1, a mutant comprising the substitution combination N35S-C57M-F70H was prepared based on the following LTTA-encoded nucleic acid:
[0201] - LTTA of Pseudomonas sp.34E 7: LTTA02, encoding nucleic acid SEQ ID NO: 3;
[0202] - LTTA of Pseudomonas sp. Irchel s3a18: LTTA03, encoding nucleic acid SEQ ID NO: 5; and
[0203] - LTTA of Chitiniphilus shinanonensis: LTTA04, encoding nucleic acid SEQ ID NO:7.
[0204] According to the method of Example 1, LTTA01 and its mutants were expressed by shaking flask culture, and enzyme powder was prepared. The activity of each wild-type LTTA and its mutants was measured according to method 2 in item vi) of Example 1, and the results are shown in Table 2, wherein the relative activity refers to the activity of the mutant / the activity of LTTA01.
[0205] Table 2
[0206] LTTA or mutan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com