Preparation method of ambroxol hydrochloride impurity F
A technology for ambroxol hydrochloride and impurities, which is applied in the field of medicinal chemistry, can solve problems such as difficult control of reaction conditions, and achieve the effects of mild reaction conditions, high yield, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
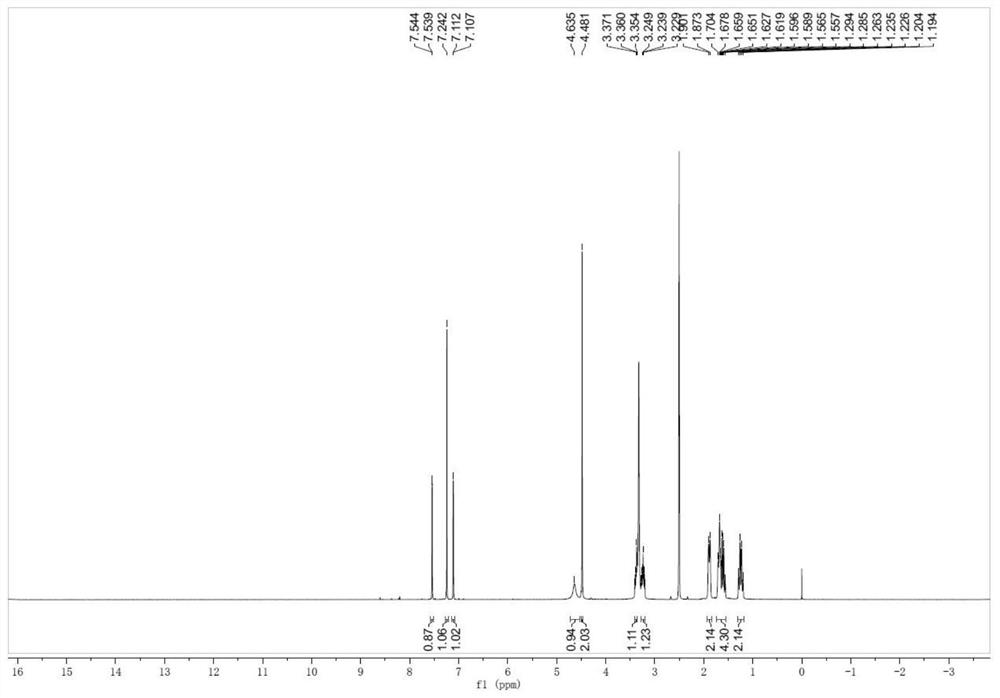
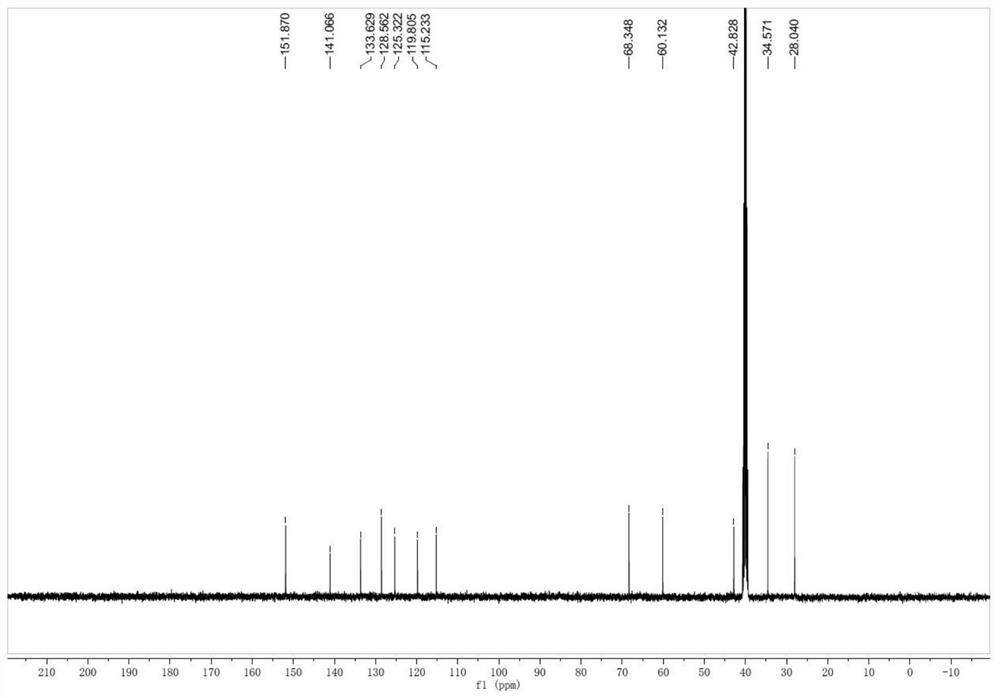
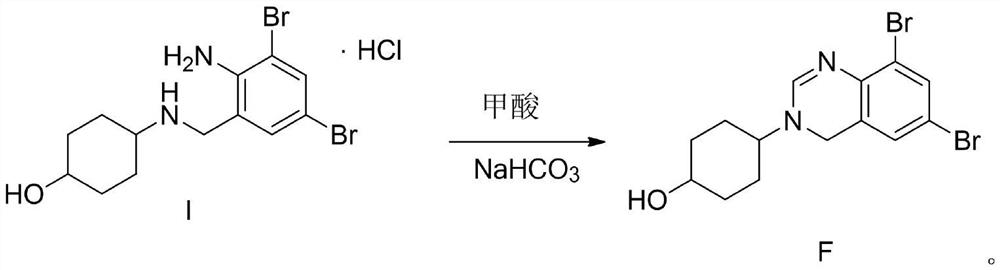
[0030] 4-(6,8-dibromo)-3,4-dihydroquinazolin-3-yl)-cyclohexanol (impurity F)
[0031] Add 40 g of ambroxol hydrochloride and 400 g of formic acid into the reaction bottle, heat at 80° C. to 90° C. for 4-6 hours, and TLC detects that the reaction is complete. Remove formic acid by rotary evaporation at 80°C to 90°C, then add 500ml of dichloromethane and 200ml of water, adjust the pH to 8-9 with saturated sodium carbonate solution, let stand to separate the liquids, dry the organic phase with anhydrous sodium sulfate, and spin to dry the solvent A solid was obtained, weighing 34.9 g, with a yield of 93.2%.
[0032] Example 1
[0033] 4-(6,8-dibromo)-3,4-dihydroquinazolin-3-yl)-cyclohexanol (impurity F)
[0034] Add 40 g of ambroxol hydrochloride and 160 g of formic acid into the reaction flask, heat at 60° C. to 70° C. for 4-6 hours, and TLC detects that the reaction is complete. Remove formic acid by rotary evaporation at 80°C to 90°C, then add 500ml of dichloromethane and 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com