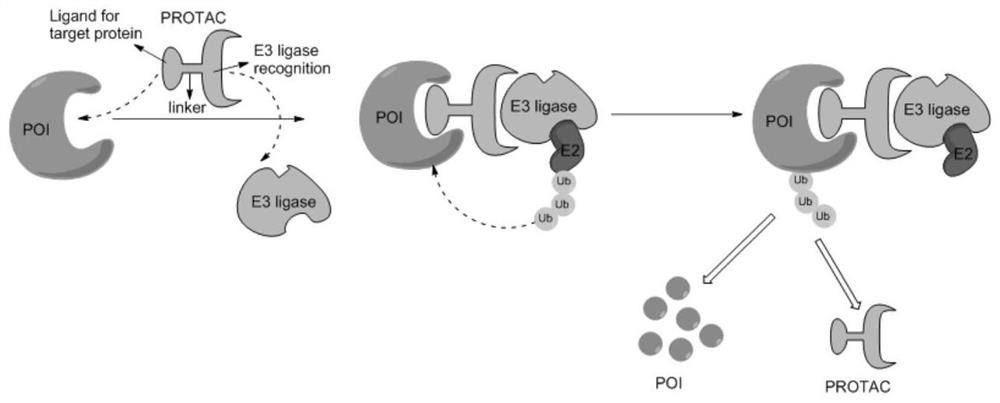
Erlotinib-based targeted degradation EGFR (epidermal growth factor receptor) protein small molecule compound as well as preparation method and application thereof
A compound and targeted technology, applied in the fields of medical preparations containing active ingredients, organic chemistry, drug combinations, etc., can solve the problems of limited inhibitory effect and drug resistance, and achieve great application prospects, good anti-tumor activity, excellent The effect of EGFR protein degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of E3 Ligand Small Molecule Compound (Compound 6)
[0050] Preparation of compound 6a:
[0051]
[0052] Compound 1a (100mmol) (n=1) was dissolved in 30mL DCM, Et 3 N (200mmol), TsCl (140mmol) was dissolved in 70mL DCM, and dropped into the reaction solution under ice-bath conditions. After overnight reaction, TLC detection (PE:EA=1:2) raw material reaction is complete, product R f = 0.3. Suction filter the reaction solution, wash the filter cake with a small amount of DCM, adjust the pH of the filtrate to neutral with 6N hydrochloric acid, add an appropriate amount of water, extract the aqueous phase twice with DCM, combine the organic phases, concentrate under reduced pressure, and obtain the compound after separation by column chromatography 2a, 40.7% yield.
[0053] Dissolve the obtained compound 2a in 100mL DMF, add NaN at room temperature 3 (100mmol), the reaction was carried out at an oil bath temperature of 100°C. After reacting f...
Embodiment 2
[0065] Embodiment 2: Preparation of PROTACs compound ALP-1
[0066]
[0067] Compound 6a (0.11 mmol) and erlotinib 7 (0.11 mmol) prepared in Example 1 were dissolved in 1 mL of DMSO, and the stirring was started. Copper sulfate pentahydrate (0.02mmol) and vitamin C (0.04mmol) were dissolved in 0.5mL water and added to the reaction system, and reacted at 50°C for 4h. It was detected by TLC (EA:MeOH=10:1) that the reaction of raw materials was complete. Added water, extracted with EA, combined the organic phases, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by column chromatography to obtain compound ALP-1 with a yield of 70%.
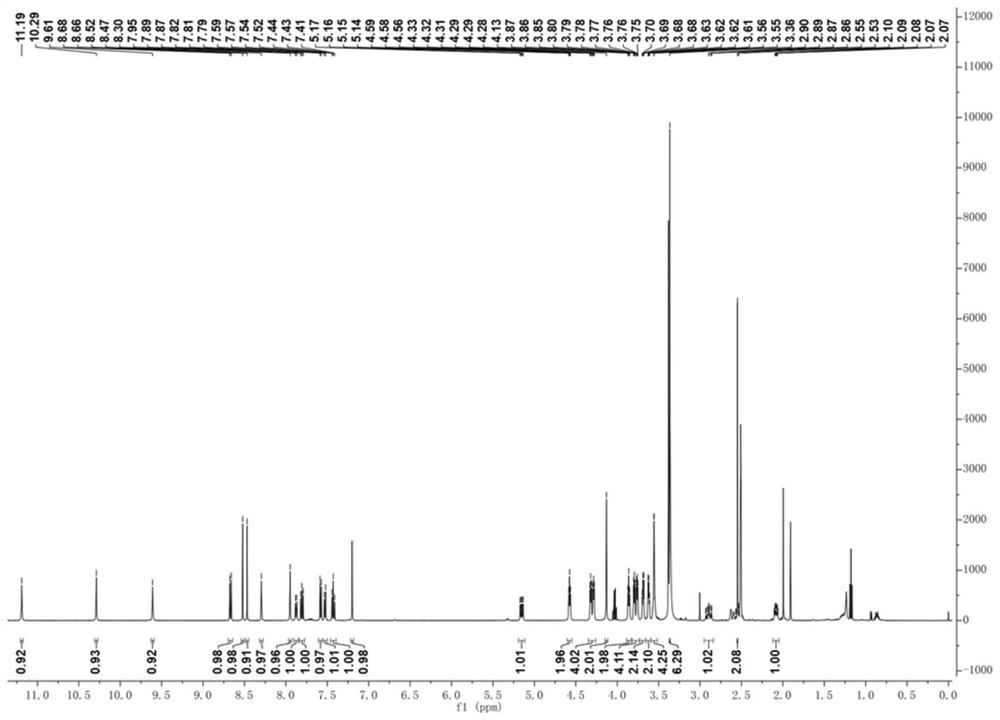
[0068] 1 H NMR (500MHz, DMSO-d 6 )δ11.19(s,1H),10.29(s,1H),9.61(s,1H),8.67(d,J=8.4Hz,1H),8.52(s,1H),8.47(s,1H), 8.30(s,1H),7.95(s,1H),7.88(d,J=8.1Hz,1H),7.84–7.77(m,1H),7.58(d,J=7.2Hz,1H),7.53(d ,J=7.7Hz,1H),7.43(t,J=7.9Hz,1H),7.20(s,1H),5.15(dd,J=12.9,5.4Hz,1H),4.58(t,J=5.1H...
Embodiment 3
[0069] Embodiment 3: Preparation of PROTACs compound ALP-2
[0070]
[0071] Compound 6b (0.35 mmol) and erlotinib 7 (0.35 mmol) prepared in Example 1 were dissolved in 2 mL of DMSO, and stirring was started. Copper sulfate pentahydrate (0.06mmol) and vitamin C (0.14mmol) were dissolved in 2mL of water and added to the reaction system, and reacted at 50°C for 5h. TLC detection (EA:MeOH=10:1) raw materials reacted completely, added water, extracted with EA, combined organic phases, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by column chromatography to obtain compound ALP-2 , yield 72%.
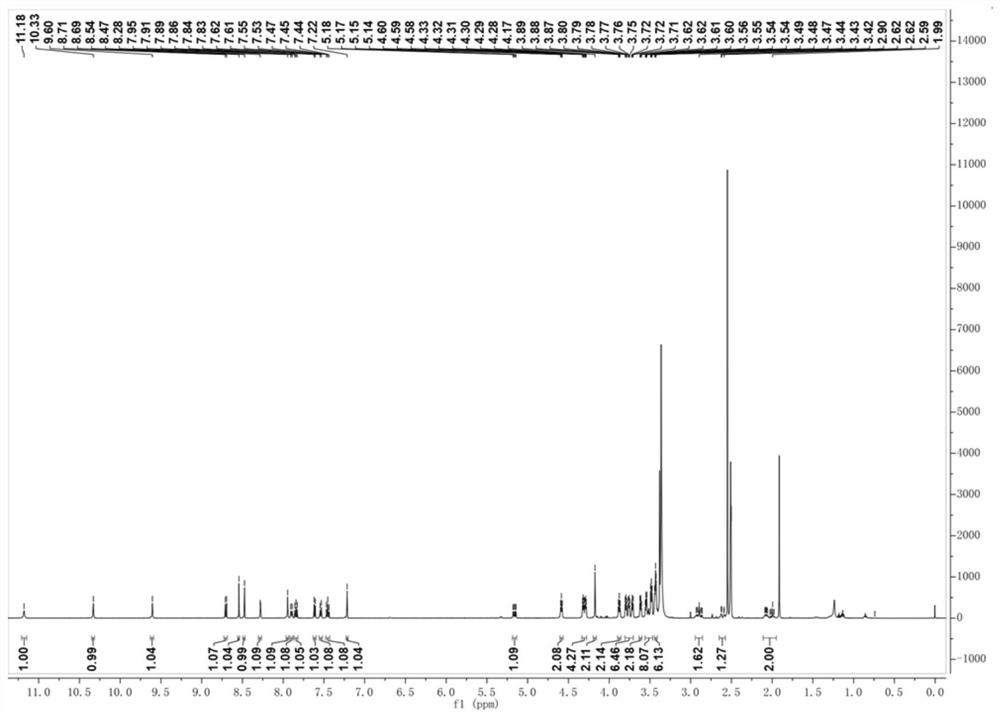
[0072] 1 H NMR(500MHz,DMSO-d6)δ11.18(s,1H),10.33(s,1H),9.60(s,1H),8.70(d,J=8.4Hz,1H),8.54(s,1H) ,8.47(s,1H),8.28(s,1H),7.95(s,1H),7.90(d,J=8.0Hz,1H),7.87–7.82(m,1H),7.61(d,J=7.3 Hz,1H),7.54(d,J=7.7Hz,1H),7.45(t,J=7.9Hz,1H),7.22(s,1H),5.16(dd,J=12.9,5.4Hz,1H), 4.59(t, J=5.1Hz, 2H), 4.30(dt, J=14.2, 4.5Hz, 4H), 4.17...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com