Composition for fixed-point modification of pAPN-gene 16 exon and application of composition
A technology of site-directed modification and composition, which can be applied to cells modified by the introduction of foreign genetic material, genetically modified cells, and the introduction of foreign genetic material using vectors, which can solve problems such as affecting the physiological functions of the body.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1 Construction of recombinant plasmid pX458-pAPN-sgRNA vector and double-stranded donor sequence design
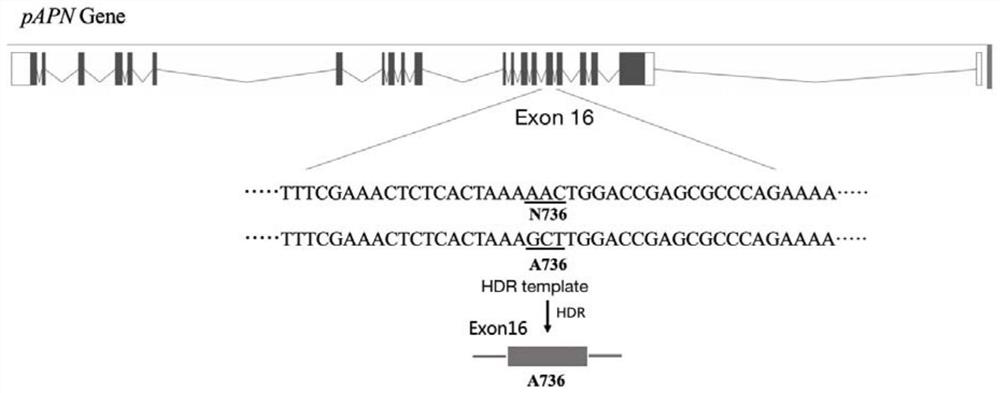
[0082]1. First lock the sequence around the 736th amino acid of the porcine pAPN gene, and use the sgRNA analysis tool CRISPOR ( crispor.tefor.net ) sgRNA selects a targeting site sgRNA close to the N736 site and has a higher score, and its sequence is shown in SEQ ID NO: 1 and SEQ ID NO: 2. Complementary paired oligonucleotide sequences as shown in SEQ ID NO: 4 and SEQ ID NO: 5; SEQ ID NO: 6 and SEQ ID NO: 7 were synthesized according to the sgRNA sequence. At the same time, according to the sgRNA sequence, this embodiment also designed a pAPN-dsODN sequence such as the dsODN sequence shown in SEQ ID NO: 3 as the double-stranded donor sequence. When the double-stranded donor sequence replaced the wild-type sequence, N736 was successfully replaced by A736. The single amino acid precise mutation pattern of porcine pAPN gene N736 is shown in the figure fig...
Embodiment 2
[0084] Example 2 Establishment and functional verification of monoclonal porcine ileal epithelial cells with precise modification of the 736th amino acid of pAPN gene
[0085] 1. Establishment of porcine ileal epithelial cells by precisely modifying the 736th amino acid of pAPN gene
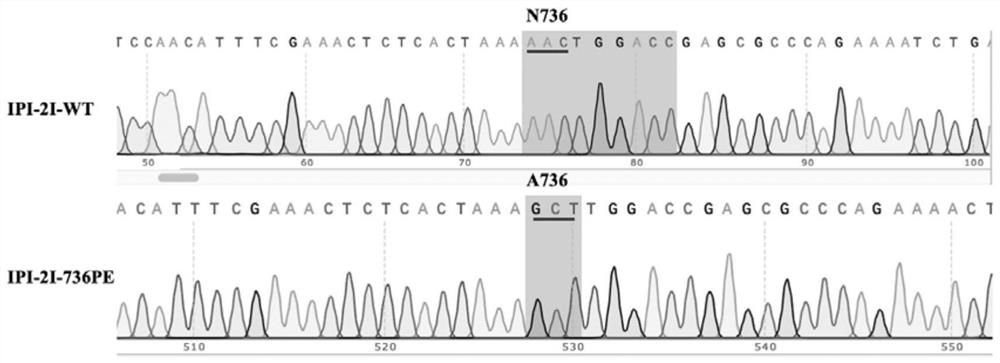
[0086] The wild-type porcine ileal epithelial cells (Immortal Pig Intestinal-2I wild type, IPI-2I-WT) were resuscitated into a 10 cm plate two days in advance, and the cells could be transfected when the cells reached 70-80% confluence. 5 μg pX458-pAPN-sgRNA-1 plasmid, 5 μg pX458-pAPN-sgRNA-2 plasmid and 5 μg pAPN-dsODN were co-transfected into IPI-2I-WT cells, and the transfection steps were strictly in accordance with the Basic Primary Nucleofector Kit (Lonza) kit manual to operate.
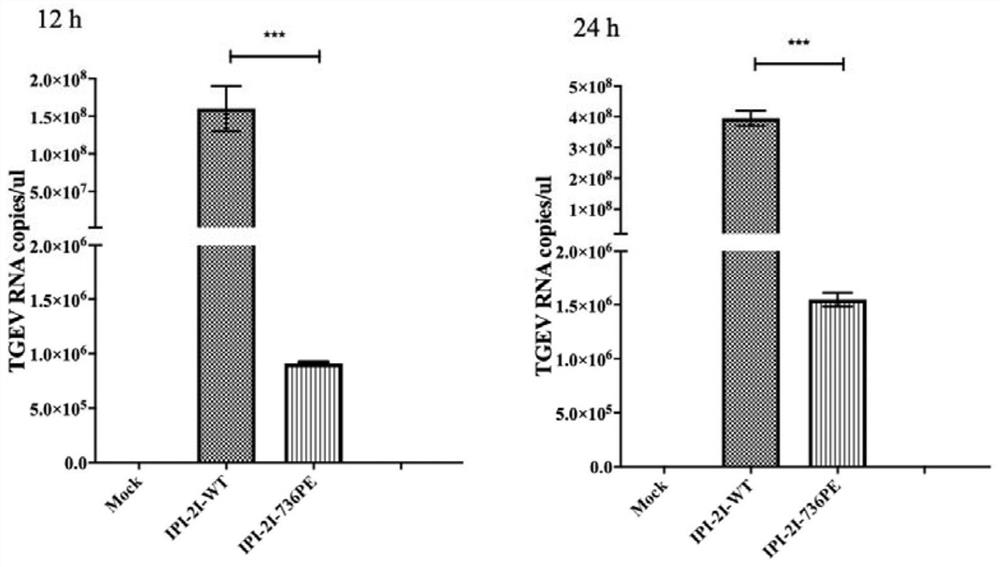
[0087] After 36 hours of electroporation, the cells were collected, and a single positive cell was sorted into a 96-well plate by a flow sorter and cultured, and the culture medium was changed every 3 days. Afte...
Embodiment 3
[0094] Example 3 Establishment of Monoclonal Porcine Fibroblasts Precisely Modified at the 736th Amino Acid of pAPN Gene
[0095] 1. Preparation of Porcine Fetal Fibroblasts
[0096] Remove the head, tail, limbs, viscera and bones from the 35-day-old pig embryo, and clean up the blood. Continue to cut the fetus with elbow ophthalmic scissors for 30 minutes to ensure that it is fully shredded. Pipette the shredded fetal tissue into a 15mL centrifuge tube with the blue tip of the scissors, add 5mL of complete medium, and remove the above solution after natural sedimentation for several minutes. And add a few drops of fetal calf serum to the lower tissue block, suck it out with a 15cm glass pasteur tube bent at the tip of 1cm, spread it in two T75 culture flasks, place the bottom of the bottle upward, and add 15mL full culture to the opposite side After 6-8 hours, carefully turn over the culture bottle, immerse the tissue pieces in the culture solution, change the solution every...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com