Pain-relieving and anti-inflammatory compound sustained-release preparation
A sustained-release preparation and technology of sustained-release preparations, applied in the direction of anti-inflammatory agents, non-central analgesics, anesthetics, etc., can solve the problems of uncertain effect strength and analgesic time, and reduce intravascular puncture and accidental acupuncture risk, ease of administration, and number of needle sticks avoided
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
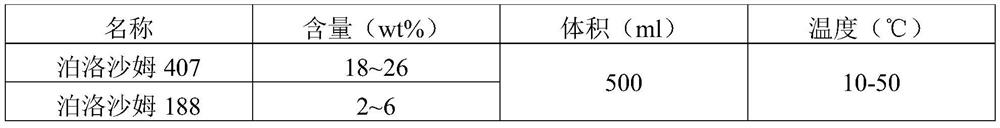
[0038] Preparation of Compositions of Poloxamer 407 and Poloxamer 188
[0039] According to Table 1, slowly add the weighed Poloxamer 407 and Poloxamer 188 into 0.1mol / L KH7.0 pH7.0 2 PO 4 In the buffer solution (room temperature), shake gently, avoid vigorous stirring, until it is completely dissolved. It appears as a colorless transparent liquid at room temperature.
[0040] Put the dissolved colorless transparent liquid into a water bath incubator to measure the viscosity at different temperatures. Do three parallel experiments.
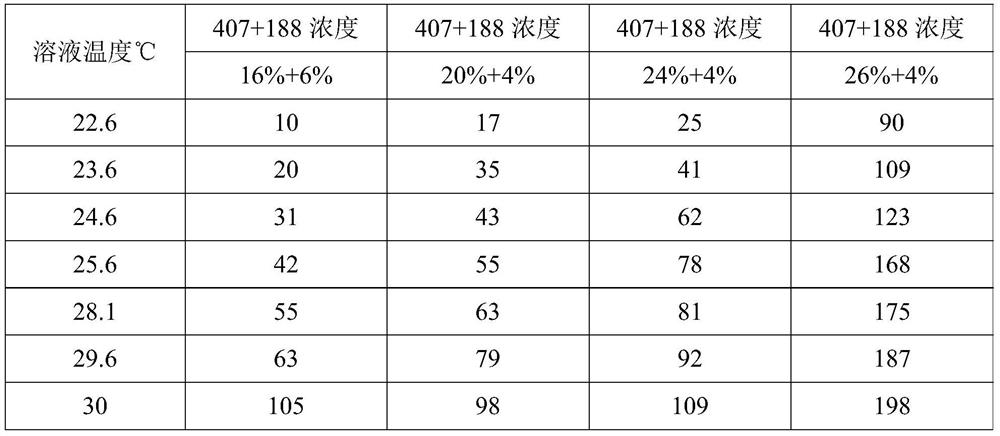
[0041] Table 1
[0042]
[0043] During the measurement process, as the viscosity increases, the model of the viscometer probe is constantly adjusted so that the opening angle is between 30% and 70%, which is convenient for obtaining relatively accurate viscosity results. The data shows that as the temperature continues to rise, High, the viscosity of the composition gradually increases, and finally reaches a semi-solid state. For the compo...
Embodiment 2
[0049] Prepare pain-relieving and anti-inflammatory compound sustained-release preparation of the present invention (without cosolvent)
[0050] According to Table 3, slowly add the weighed poloxamers 407 and 188 into 0.1mol / L KH7.4 pH7.4 2 PO 4 In the buffer solution (room temperature), shake gently, avoid vigorous stirring, until it is completely dissolved. It appears as a colorless transparent liquid at room temperature. Add different concentrations of ropivacaine and meloxicam into the solution, heat to 80-90°C to mix, stir for 30 minutes, cool down to 2-8°C, subpackage, and sterilize at 121°C for 12 minutes.
[0051] table 3
[0052]
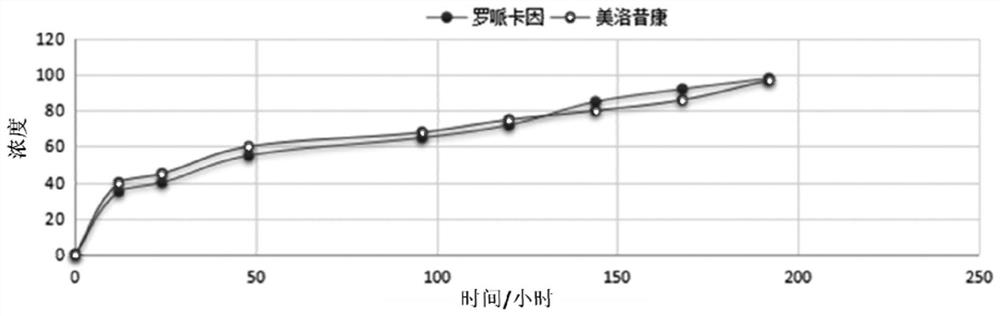
[0053] After measurement, the sterilized preparations of ropivacaine and meloxicam under different concentrations, the higher the concentration of main drug, the lower the viscosity of the preparation. At the same time, 8% and 4% ropivacaine, 0.24% and 0.12% At the concentration of meloxicam, after the formulation was left standing a...
Embodiment 3
[0055] Prepare pain-relieving and anti-inflammatory compound sustained-release preparation (containing cosolvent) of the present invention
[0056] According to Table 4, slowly add poloxamer 407 and 188 which have been weighed respectively into 0.1mol / L KH with pH7.4 2 PO 4 In the buffer solution (PB), shake gently, mix well, divide into 16 parts on average, and mark them as experiments 1-16 in turn, and then place the 16 parts of preparations in a water bath at 90°C for 10 minutes and heat the weighed merlot Add four preparations of oxicam, ropivacaine and DMSO, use PB to make up the volume to the required volume; continue to heat and stir for 10 minutes to make it evenly mixed, then cool down to 2-8°C, subpackage, and extinguish at 121°C. Bacteria 12min. Among them, the concentration of DMSO was 10-20%, ropivacaine 4%, and meloxicam 0.12%, and the orthogonal test was carried out. (the stated content is the mass percent content)
[0057] Table 4
[0058]
[0059]
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com