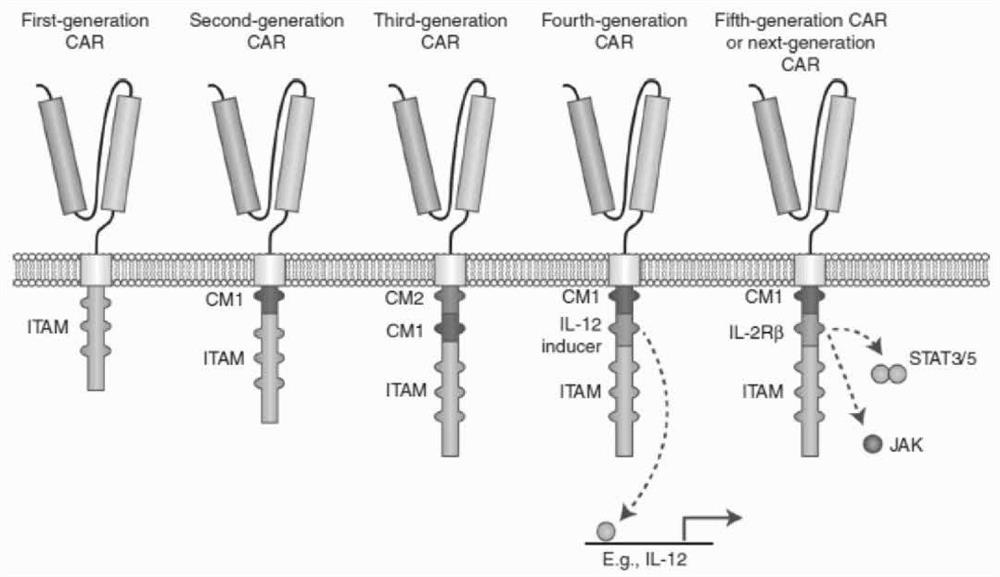
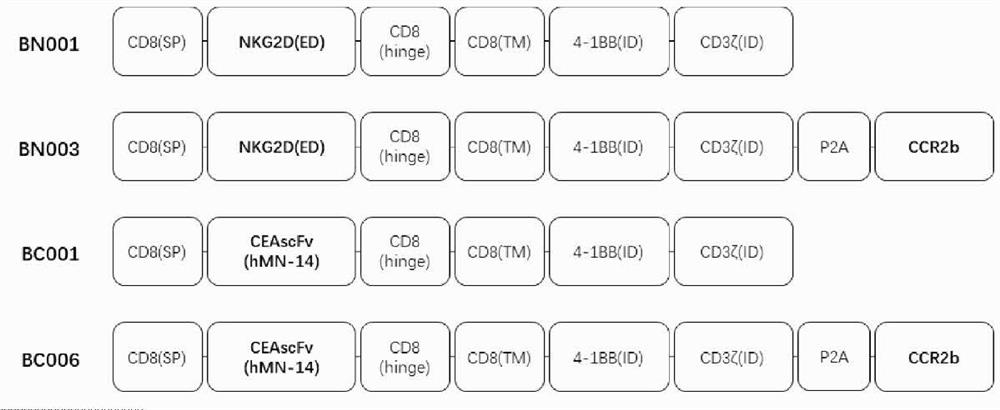
Engineered immune cell for combined expression of CCR2b as well as preparation and application of engineered immune cell
An immune cell and engineering technology, applied in the field of tumor immunity and cell therapy, can solve problems such as excessive release of cytokines, recurrence, and failure to achieve therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0259] Example 1 Lentivirus preparation
[0260] 1.1 Obtaining lentiviral vector plasmids
[0261] The nucleotide sequences of BN001, BN003, BC001, and BC006 were synthesized from the whole gene, and then connected to the lentiviral vector pCDH-EF1-MCS-T2A-copGFP plasmid by molecular cloning, so that it was in the human EF1α promoter and Kozak sequence. expression under control.
[0262] Transfection of lentiviral vector plasmid into 293T cells
[0263] Mix the above-mentioned lentiviral vector plasmids with lentiviral packaging plasmids pMD2.G, pRSV-Rev and pMDLg / pRRE with polyethyleneimine transfection reagent, and co-transfect 293T cells. After culturing for 48 h, the virus supernatant was collected separately, centrifuged at 4500 rpm at 4°C for 10-15 min, filtered through a filter membrane with a pore size of 0.5 μm, and then concentrated with a hollow fiber column ultrafiltration system, and then purified by chromatography. The lentivirus was purified, and finally fi...
Embodiment 2
[0268] Example 2 Preparation and detection of CAR-T cells
[0269] 2.1 T cell preparation
[0270] Adjust the density of peripheral blood mononuclear cells from healthy donors to 2 × 10 6 / ml, add 50 ng / ml anti-CD3 antibody, 50 ng / ml anti-CD28 antibody, and 200 IU / ml recombinant IL-2, and culture in a cell incubator for 24 h (cultivation temperature is 37°C, carbon dioxide concentration is 5%).
[0271] lentiviral transduction of T cells
[0272] Wash the obtained T cells and adjust the cell density to 2 × 10 6 / ml. Add lentivirus at MOI = 1-10 TU / cell for transduction, supplement 50 ng / ml anti-CD3 antibody, 50 ng / ml anti-CD28 antibody, and 200IU / ml recombinant IL-2, and place in the cell culture incubator Medium culture (cultivation temperature is 37°C, carbon dioxide concentration is 5%). After 24 h, adjust the cell density to 1.5-2 × 10 6 / ml, and supplemented with 300 IU / ml of IL-2. On the 4th day after transduction, wash the cells to remove residual lentiviral p...
Embodiment 3
[0282] Example 3 Target cell detection
[0283] 3.1 Target cell culture conditions
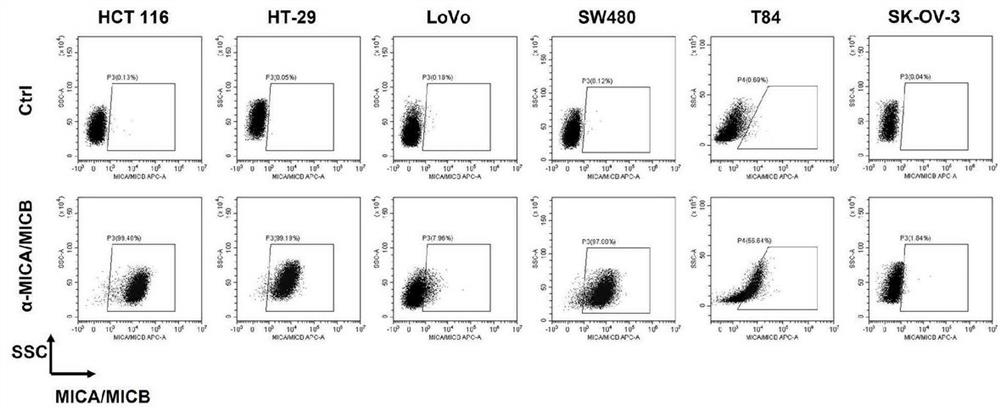
[0284] Tested colorectal cancer cell lines (also known as target cells or target cell lines): HCT 116 (McCoy's 5a medium + 10% fetal bovine serum + 100 U / ml penicillin + 100 μg / ml streptomycin), HT-29 (McCoy's 5a medium + 10% fetal bovine serum + 100 U / ml penicillin + 100 μg / ml streptomycin), LoVo (F-12K medium + 10% fetal bovine serum + 100 U / ml penicillin + 100 μg / ml streptomycin), SW480 (Leibovitz's L-15 medium + 10% fetal bovine serum + 100 U / ml penicillin + 100 μg / ml streptomycin), T84 (DMEM / F-12 medium + 5% fetal bovine serum + 100 U / ml penicillin + 100 μg / ml streptomycin), SK-OV-3 (McCoy's 5a medium + 10% fetal bovine serum + 100 U / ml penicillin + 100 μg / ml streptomycin).
[0285] Ligand (MICA / MICB) expression detection
[0286] The above target cells were washed twice with PBS and resuspended with FACS buffer. Add APC-labeled anti-human MICA / MICB antibody to each target cell sus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com