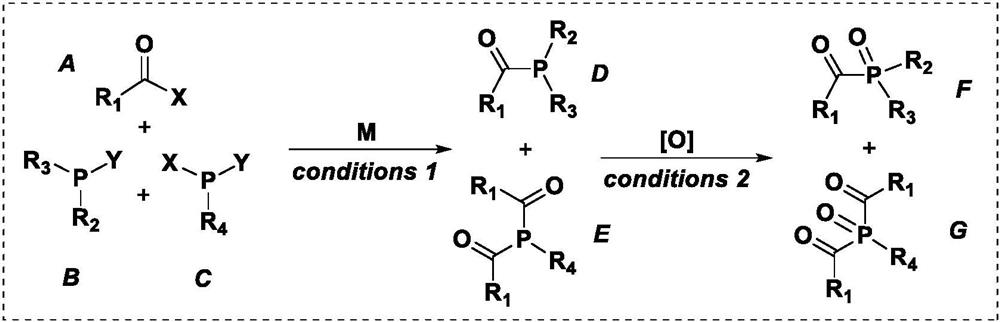
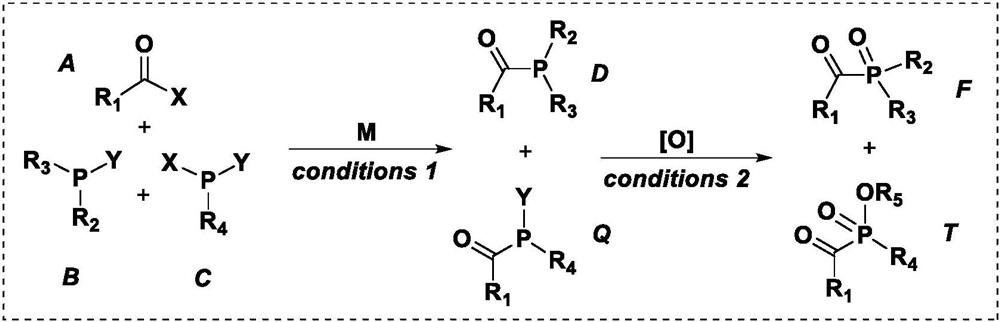
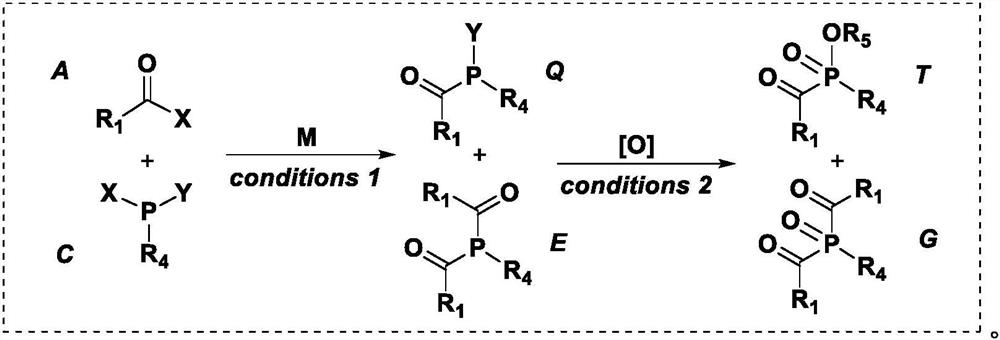
Co-production preparation of monoacylphosphine and bisacylphosphine and oxides thereof
A technology of bisacylphosphine oxide and monoacylphosphine is applied in the field of co-production preparation of monoacylphosphine, bisacylphosphine and their oxides, and can solve the problem that monoacylphosphine oxide products cannot be prepared by application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Coproduction of TPO and 819
[0046] Under nitrogen protection and room temperature, add 1.4 grams of zinc powder, 15 milliliters of ethyl acetate, 4.0 grams of 2,4,6-trimethylbenzoyl chloride to the 100 milliliter reaction flask successively under stirring, and then add to the mixture A mixture of 1.08 g of phenylphosphorous dichloride and 1.32 g of diphenylphosphorous chloride in 5 ml of ethyl acetate was added dropwise, and the temperature was maintained for 2 hours after the addition was complete. After the reaction is completed, add 15 ml of water and 1.6 ml of 30% hydrogen peroxide to the system respectively, and continue the reaction for half an hour. Column chromatography separated 1.7 g of 819 (68% yield) and 1.9 g of TPO (91% yield).
[0047] The above reaction was repeated but the solvent was changed to dimethyl carbonate to obtain 1.5 grams of 819 and 1.8 grams of TPO;
[0048] The above reaction was repeated but the zinc dust was changed to mag...
Embodiment 2
[0049] Embodiment 2: Coproduction of TPO and 819
[0050] Under nitrogen protection and room temperature, add 17.2 kilograms of zinc powder, 200 liters of ethyl acetate, 49 kilograms of 2,4,6-trimethylbenzoyl chloride to a cubic enamel reaction kettle, and then drop 13.3 kilograms of The mixture of phenylphosphorous dichloride and 16.2 kilograms of diphenylphosphorous chloride in 80 liters of ethyl acetate continued to be incubated for 6 hours after the dropwise addition was completed. After the reaction is completed, add 180 liters of water and 20 liters of 30% hydrogen peroxide to the system respectively, and continue the reaction for half an hour. 18.7 kg of 819 and 23.1 kg of TPO were separated by beating.
[0051] Wherein, 1.1 kg of monoacyl bisphosphine oxide H was isolated simultaneously. HRMS high-resolution mass spectrometry data: molecular formula C 28 h 27 o 3 P 2 (M+H): theoretical value 473.1435, experimental value 473.1441; molecular formula C 28 h 26 o ...
Embodiment 3
[0052] Example 3: Preparation of macromolecular polymeric bisacylphosphine oxide
[0053] Under nitrogen protection and room temperature, 1.3 grams of zinc powder and 15 milliliters of ethyl acetate were added successively to a 100 milliliter reaction flask, and then 3.8 grams of pre-prepared 3,3'-methylene-bis(2, 4,6-trimethylbenzoyl chloride) and 1.8 grams of freshly distilled phenylphosphorous dichloride mixture in 5 milliliters of ethyl acetate, continued to keep warm for 2 hours after the dropwise addition was completed. After the reaction was completed, 15 ml of water and 1.4 ml of 30% hydrogen peroxide were added to the system respectively, and the reaction was continued for half an hour. Separation by beating in ice-cold heptane yielded 2.3 g of product K as a yellow amorphous powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com