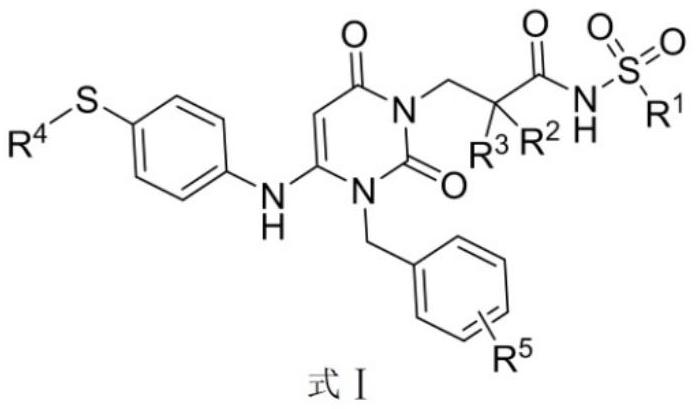
Dihydropyrimidine compound and preparation method and application thereof
A compound and solvate technology, applied in the field of medicinal chemistry, can solve the problems of failure to reach the main efficacy endpoint and high incidence of taste-related adverse events, and achieve the effects of good affinity, easy operation and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
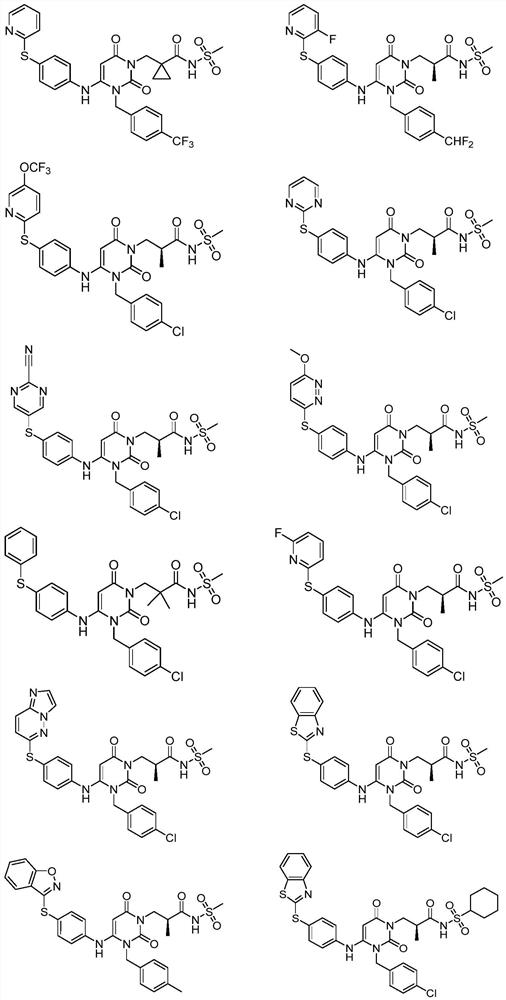
Image
Examples
Embodiment 1
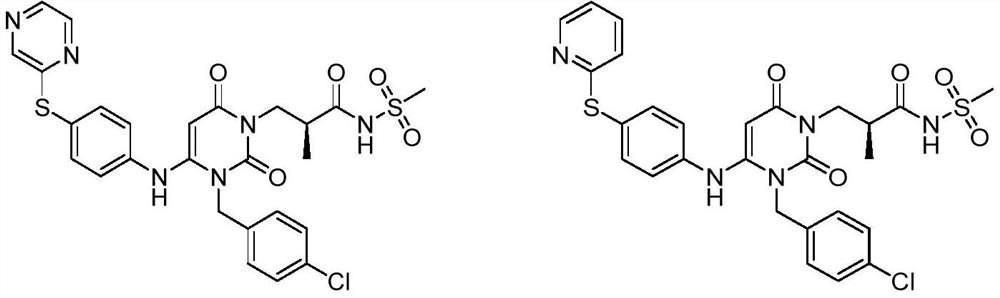
[0052] Example 1: (S)-3-(3-(4-chlorobenzyl)-2,6-dioxa-4-(4-(pyrazin-2-ylthio)phenyl)amino)- Preparation of 3,6-dihydropyrimidin-1(2H)yl)-2-methyl-N-(methylsulfonyl)propionamide (compound 1):
[0053]
[0054] Step 1: Preparation of 4-(pyrazine-2-thio)aniline (compound b-1)
[0055] 2-Fluoropyrazine (51.0g, 0.52mol) and p-aminothiophenol (61.3g, 0.49mol) were dissolved in dimethyl sulfoxide (360ml), cesium carbonate (320g, 0.98mol) was added to obtain a reaction mixture, And the reaction mixture was stirred at a constant speed with a mechanical stirrer. Then the internal temperature of the reaction system was raised to 80° C. for 2 h. The progress of the reaction was tracked by thin-layer chromatography. After the reaction was complete, the reaction mixture was added to three times the volume (about 1 L) of water and kept stirring. Then extract the product three times with ethyl acetate, combine and dry the ethyl acetate and then concentrate to obtain the crude product. T...
Embodiment 2
[0075] Example 2: (S)-3-(3-(4-chlorobenzyl)-2,6-dioxa-4-(4-(pyridin-2-ylthio)phenyl)amino)-3 , Preparation of 6-dihydropyrimidin-1(2H)yl)-2-methyl-N-(methylsulfonyl)propionamide (compound 2)
[0076] Compared with the preparation method of Example 1, the preparation method of this example is replaced by equimolar 2-fluoropyridine in step 1, and the rest of the conditions are the same, and the compound 2 is obtained as a white solid, with a yield of 76.0% , with a purity of 99.05%.
[0077] ESI-MS:m / z=600.1(M+H) + .
[0078] 1 H NMR (400MHz, DMSO-d6)δ11.70(s,1H),8.80(s,1H),8.31(dd,J=5.0,2.0Hz,1H),7.99–7.89(m,1H),7.45– 7.37(m,2H),7.30(d,J=8.4Hz,2H),7.25(m,4H),7.14–7.09(m,1H),7.03(d,J=8.3Hz,1H),5.42–5.15 (m,2H), 4.62(s,1H), 3.88(m,2H), 3.01(s,3H), 2.69(m,1H), 0.98(m,3H).
Embodiment 3
[0079] Example 3: 1-(2,6-dioxo-4-(4-(pyridine-2-thiophenyl)amino)-3-(4-(trifluoromethyl)benzyl)-3,6 - Preparation of dihydropyrimidin-1(2H)ylmethyl)-N-(methylsulfonyl)cyclopropane-1-carboxamide (Compound 3)
[0080] Compared with Example 1, the preparation method of this example replaces 2-fluoro-pyrazine with equimolar 2-fluoropyridine in step 1, and replaces (S)-3-hydroxyl-2-methyl Methyl propionate was replaced by equimolar methyl 1-(hydroxymethyl)cyclopropane-1-carboxylate, and the rest of the conditions were the same to obtain compound 3 as a white solid with a yield of 76.1% and a purity of 99.05%.
[0081] ESI-MS:m / z=646.1(M+H) + .
[0082] 1 H NMR (400MHz, DMSO-d6) δ11.40 (s, 1H), 8.89 (s, 1H), 8.35 (dd, J = 5.2, 2.0Hz, 1H), 7.85 (m, 1H), 7.46–7.38 ( m,2H),7.35(d,J=8.4Hz,2H),7.23–7.15(m,4H),7.15–7.09(m,1H),7.04(d,J=8.3Hz,1H),5.28(s ,2H),4.65(s,1H),4.13(s,2H),3.12(s,3H),1.07–0.92(m,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com