Method for detecting amantadine compounds and triazine herbicides in algae
A technology for the detection of amantadine and its detection method, which is applied in the field of detection of amantadine compounds and triazine herbicides in algae, and can solve the problems of simultaneous detection of two types of drugs, cumbersome operation, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Sample pretreatment
[0127] Weigh 3.0g (±0.01g) of the algae sample to be tested, homogenize it with a homogenizer, wash the knife head with 15mL acetonitrile, add it to the sample, vortex at 2000r / min for 30s, extract it ultrasonically for 30min, and centrifuge at 8000r / min for 10min Take 5mL supernatant in a 15mL centrifuge tube, dry it with nitrogen at 40°C, add 1mL of acetonitrile aqueous solution containing 0.1vol% formic acid (the volume ratio of acetonitrile and water in the acetonitrile aqueous solution is 6:4) 1mL, 50mg PSA adsorption (particle size: 50-75μm), vortex at 2000r / min for 1min, centrifuge at 5000r / min for 10min, take the supernatant and pass through a 0.22μm PVDF membrane to obtain the solution to be tested.
[0128] Parameters for UHPLC include:
[0129] Liquid phase model: Dionex UltiMate 3000 ultra-high performance liquid phase system
[0130] Column: Waters BEH C 18 (2.1mm×100mm 1.7μm);
[0131] Column temperature: 45°C;
[0132] Mobile phas...
Embodiment 2
[0165] Choice of extractant
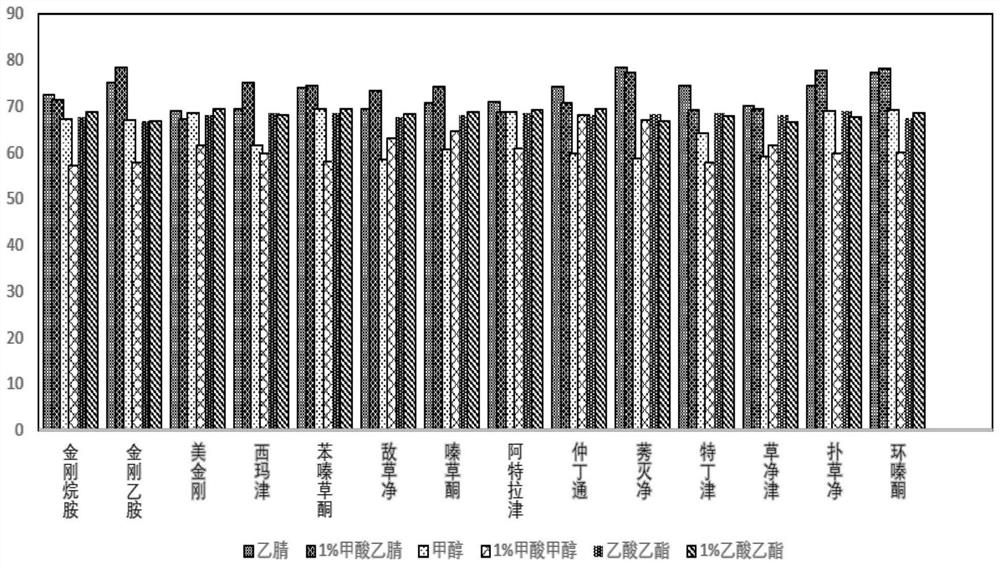
[0166] The extractant acetonitrile in Example 1 was replaced with 1 vol% formic acid acetonitrile, methanol, 1 vol% formic acid methanol, ethyl acetate, 1 vol% formic acid ethyl acetate to obtain an extract.
[0167] Utilize formula 1 to calculate the extraction rate in the extract solution:
[0168]
[0169] In Formula 1:
[0170] Instrument detection value: adding standard solution to blank matrix, after pretreatment, the test result on the machine, μg / L;
[0171] Blank sample matrix addition value: the value of the standard solution added to the blank matrix sample, μg / L.
[0172] The corresponding extraction rate of each extractant obtained is as follows: figure 1 shown. From figure 1 It can be seen that the extraction efficiency of acetonitrile is close to the effect of 1vol% formic acid acetonitrile, ethyl acetate and 1vol% formic acid ethyl acetate, indicating that acetonitrile, 1vol% formic acid acetonitrile, ethyl acetate and 1vol...
Embodiment 3
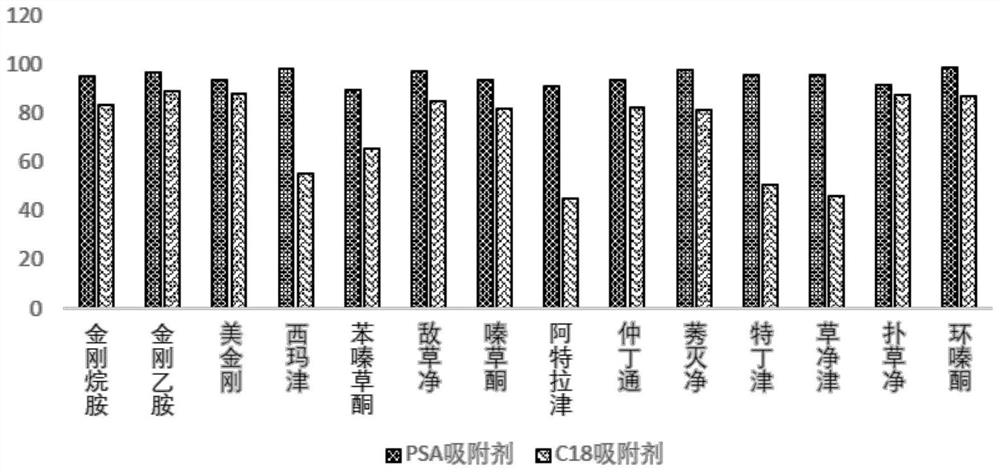
[0174] Sorbent selection
[0175] The PSA adsorbent in embodiment 1 is replaced by C 18 Adsorbent, calculate recovery using Equation 2:
[0176]
[0177] In formula 2:
[0178] On-machine test results: After the blank matrix sample is pre-treated, the standard solution is used to constant volume, and the test results on the machine after the adsorbent is purified, μg / L;
[0179] The value of matrix calibration: After the blank matrix sample is pre-treated, use a certain concentration of standard solution to make up the volume, μg / L.
[0180] Effect of different adsorbents on the recovery rate of adamantane compounds and triazine herbicides figure 2 shown. From figure 2 It can be seen that: C 18 Sorbent has certain adsorption to part herbicide class medicine, causes recovery rate to reduce, therefore, the present invention mainly adopts PSA sorbent as purification means, C 18 As an auxiliary means, sorbent purification is only used when dealing with biological sampl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com