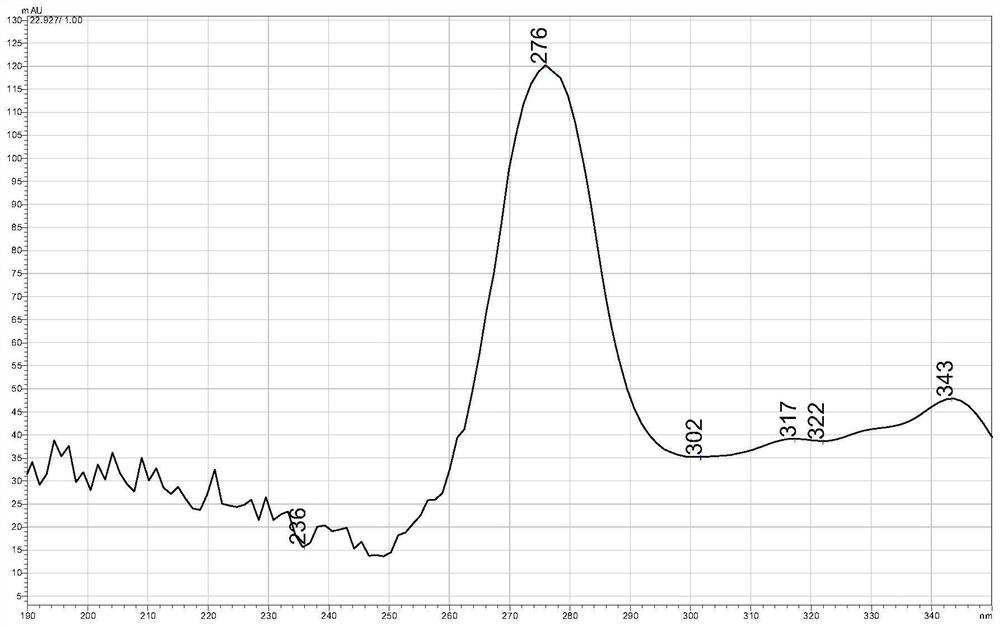
Resolution method of prulifloxacin enantiomers
A technology of prulifloxacin and enantiomers, applied in material separation, material analysis, measuring devices, etc., can solve the problem of no split report and achieve good linear relationship and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Effect of Diluent and Injection Volume on Resolution
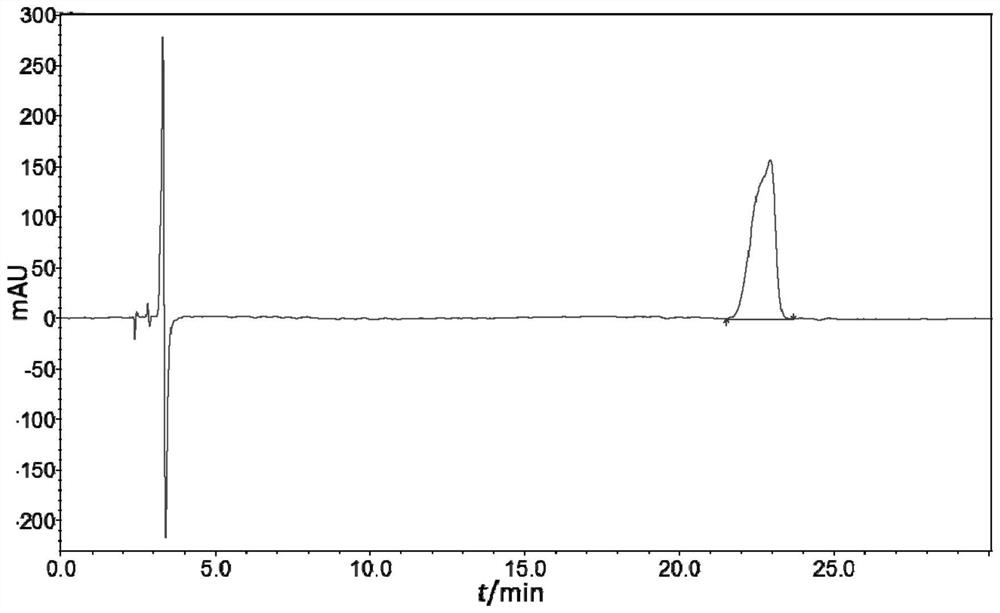
[0039] Take acetonitrile as the reference substance sample solution one (solution b) with a concentration of 206.4 μg / mL as the diluent. When the injection volume is 10 μL, the peak shapes of the two enantiomers of prulifloxacin are particularly poor. All unsatisfactory, and have bigger solvent peak. Take acetonitrile as the reference substance stock solution (solution a) with a diluent concentration of 1032.1 μg / mL and reference substance sample solution 2 (solution d) with a mobile phase as a diluent concentration of 200.2 μg / mL, and inject 2 μL and 10 μL respectively , the separation between the two enantiomer peaks of prulifloxacin injected by the two methods is about 3.61 and 3.78, and there is almost no difference in the peak shape of prulifloxacin, except that the solvent peak is larger when acetonitrile is used as the diluent, see the chromatogram figure 1 .
[0040] It can be seen that when the...
Embodiment 2
[0041] Example 2: Effect of Diluent on Stability
[0042]Respectively take the reference substance stock solution (solution a) with acetonitrile as the diluent concentration of 1032.1 μg / mL and the reference substance sample solution 2 with the mobile phase as the diluent concentration of 200.2 μg / mL just prepared and after standing for a period of time. (Solution d), according to "Experimental Reagents and Detection Method 3 Chromatographic Conditions", sample injection analysis, wherein, the injection volume of solution a is 2 μL, and the injection volume of solution d is 10 μL, and the stability of the solution is investigated.
[0043] Results: When diluting with mobile phase, the peak area of the two enantiomers of prulifloxacin decreased by about 2% after being placed for 3 hours, and the peak area of the enantiomer of prulifloxacin peak decreased by <0.2% when diluted with acetonitrile for 80 h, almost no Variety.
[0044] It can be seen that the stability of pruli...
Embodiment 3
[0045] Example 3: Effect of Chiral Mobile Phase Additives on Resolution
[0046] The enantiomers of prulifloxacin were separated and analyzed by using L-phenylalanine, L-isoleucine and L-proline as chiral ligands, among which, the selected concentration was about 1032.1 μg / mL For the prulifloxacin reference stock solution (solution a), the injection volume was 2 μL for the experiment.
[0047] Results: When L-proline was used, prulifloxacin had only one chromatographic peak, and the enantiomers of prulifloxacin could not be resolved; while L-phenylalanine and L-isoleucine were used in the chromatogram Prulifloxacin has 2 chromatographic peaks, which can realize the separation of prulifloxacin enantiomers. The retention time of the enantiomer of prulifloxacin is longer when L-phenylalanine is resolved, and the proportion of methanol is increased to 36% to investigate the separation between the two enantiomer peaks when the retention time of the enantiomer of prulifloxacin is s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com