Adult liver progenitor cells for treating acute-on-chronic liver failure
A technology for acute liver failure and progenitor cells, applied in animal cells, organic active ingredients, vertebrate cells, etc., can solve problems such as coagulation factor consumption and severe bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Embodiment 1: the preparation of HALPC
[0158] HALPC were prepared from livers of healthy cadaveric or beating donors as described in EP 3140393 or WO2017 / 149059. Briefly, hepatocyte preparations were resuspended in Williams'E medium supplemented with 10% FBS, 10 mg / ml INS, 1 mM DEX. primary cells in Flasks were incubated at 37 °C in 5% CO 2 cultivated in a completely humidified atmosphere. After 24 hours, the medium was changed to remove unattached cells, and refreshed twice a week thereafter while the cultures were followed microscopically daily. After 12-16 days the medium was changed to high glucose DMEM supplemented with 9% FBS. Cell types with mesenchymal-like morphology emerge and proliferate. When reaching 70-95% confluency, trypsinize cells with recombinant trypsin and 1 mM EDTA and dilute at 1-10x10 3 cells / cm 2 Density re-plated. At each passage, cells were trypsinized when they were 80-90% confluent.
[0159] Tests on the cells confirmed that they...
Embodiment 2
[0160] Example 2: Administration of HALPC to patients (interim results)
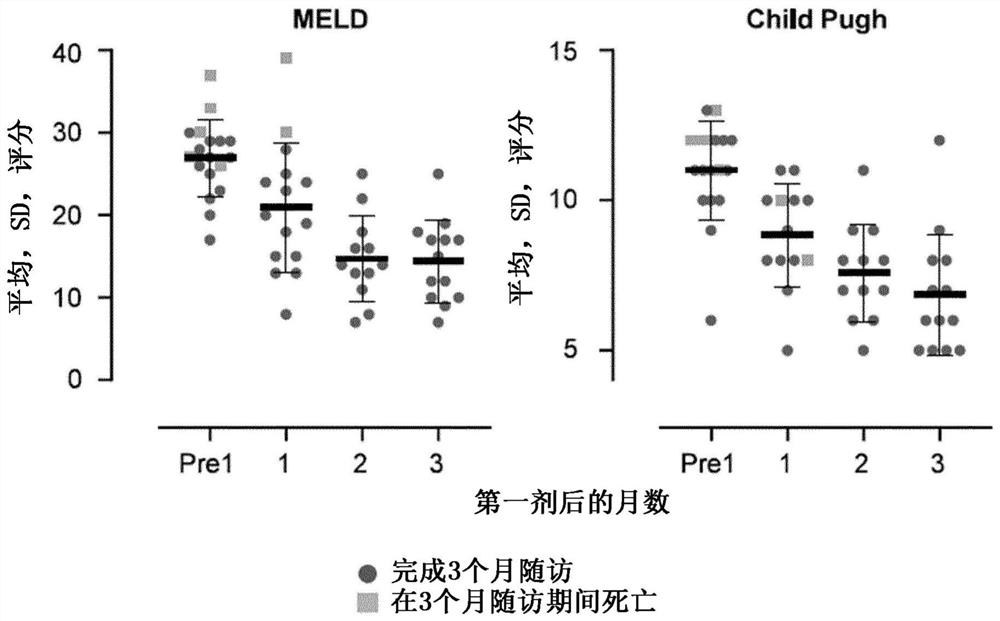
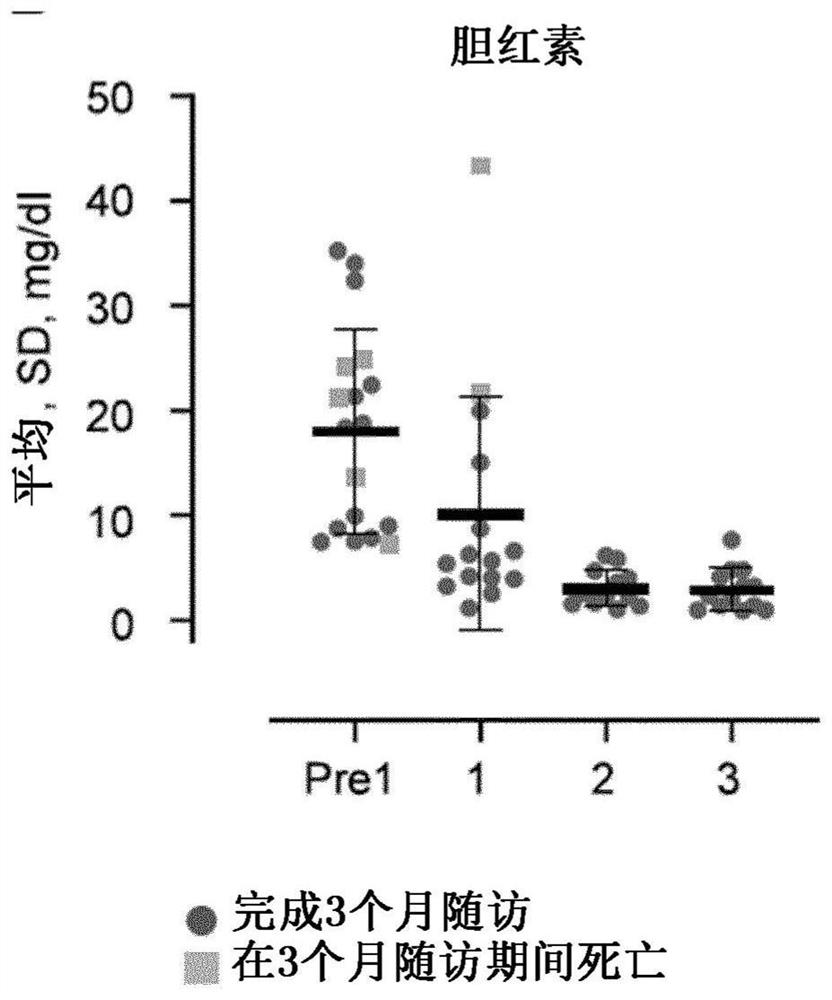
[0161] Eight patients with confirmed acute-on-chronic liver failure (ACLF) and seven patients with acute decompensation (AD) at risk of developing ACLF were treated with HALPC prepared according to Example 1 using the dosage regimen of the present invention. Cells were counted using the manual method described above. The MELD scores of the patients before treatment ranged from 18 to 35, with an average of about 27. Serum concentrations of total bilirubin were greater than 6 mg / dL (>100 umol / L) in each patient; between patients, they ranged from about 7 to about 43 mg / dL, with a mean of about 22 mg / dL. All patients received standard medical care (SMT) as required by their clinical status, but without concomitant anticoagulant therapy.
[0162] For each administration of HALPC, the vial containing the cells prepared according to Example 1 was thawed and diluted with 45 mL of a sterile liquid vehicle cont...
Embodiment 3
[0168] Example 3: Administration of HALPC to patients (full results)
[0169] The clinical trial of Example 2 was continued until a total of 22 patients were treated with the present invention. Cell counts and doses for this example were provided by the automated method described above using a Nucleocounter NC-200. In summary, patients are treated as follows:
[0170] One patient did not receive cells due to technical issues. Three patients received one infusion of approximately 600,000 cells / kg. Three additional patients received two infusions of approximately 600,000 cells / kg approximately 7 days apart. Three additional patients received a single infusion of approximately 800,000 cells / kg. Four patients received one infusion of approximately 1,200,000 cells / kg. Eight patients received two infusions of approximately 1,200,000 cells / kg approximately 7 days apart. In all cases, the infused cells were contained in a composition containing only pharmacologically insignifica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com