Preparation method of 4-ethylbenzyl chloride
A technology of ethylbenzyl chloride and ethylbenzene, which is applied in the field of preparation of 4-ethylbenzyl chloride, can solve the problems of being unsuitable for industrial production, and achieve the effects of convenient industrial scale-up production, fewer isomers, and simple synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
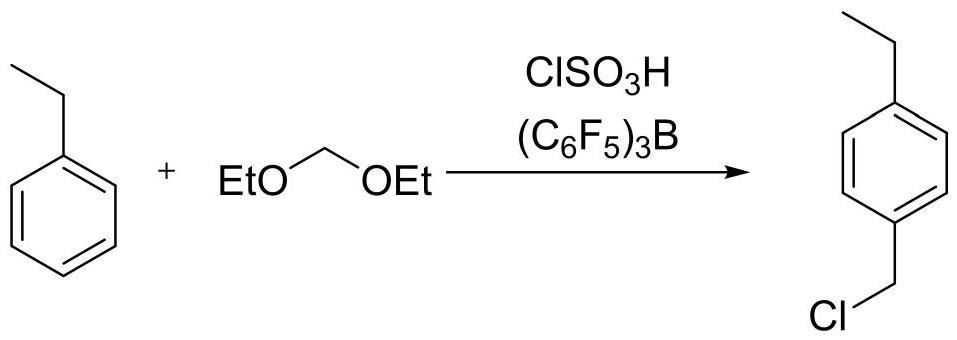
[0029] Under the condition of nitrogen flow protection, 10.3g (0.02eq) of tris(pentafluorophenyl)borane, 67g (1.15eq) of chlorosulfonic acid and 400mL of dichloromethane were successively added to the reaction flask, and the temperature was lowered to -5°C , slowly dropwise add acetal 57.3 (1.1eq), react for 30 minutes after the dropwise addition, slowly add 53g (0.5mol, 1eq) ethylbenzene dichloromethane solution dropwise at -5 ~ 0°C, and react after the dropwise addition After 1 hour, 2% of the remaining raw material was detected by GC, and the ratio of 4-ethylbenzyl chloride to 2-ethylbenzyl chloride was 94%:6%. Add 150 g of water to quench, separate layers, extract the aqueous phase with 100 mL of dichloromethane, combine the organic phases, wash once with 100 mL of 8% sodium bicarbonate, dry the organic phase with sodium sulfate, filter, and concentrate the filtrate under reduced pressure to remove most of the solvent , add n-heptane to replace, filter, the fil...
Embodiment 2
[0032] Under the condition of nitrogen flow protection, 0.205Kg of tris(pentafluorophenyl)borane, 2.80Kg of chlorosulfonic acid and 20L of dichloromethane were successively added to the reaction flask, the temperature was lowered to -5°C, and acetal 2.35 kg, reacted for 30 minutes after the dropwise addition, slowly added 2.12Kg ethylbenzene dichloromethane solution dropwise at -5~0°C, reacted for 1 hour after the dropwise addition, GC detected 1.7% of the remaining raw materials, added 15Kg water to quench, Separate the layers, extract the aqueous phase with 5L of dichloromethane, combine the organic phases, wash once with 8% sodium bicarbonate 8Kg, dry the organic phase with sodium sulfate, filter, concentrate the filtrate under reduced pressure to remove most of the dichloromethane, add 10L of normal Heptane was replaced, concentrated to the remaining 6L, cooled to 5-10°C, filtered, the filter cake was recovered tris(pentafluorophenyl)borane, and the filter cake was dried to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com