Hyaluronic acid photo-induced carrier as well as preparation method and application thereof
A technology of hyaluronic acid and sodium hyaluronate, which is applied in the direction of pharmaceutical formulations, cosmetic preparations, dressing preparations, etc., can solve the problems of low utilization rate, lack of biocompatibility, poor water solubility, etc. Skin damage, convenience-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The embodiment of the present invention provides a method for preparing a hyaluronic acid photoinducible carrier, comprising the following steps:
[0029] S1, using hyaluronidase to hydrolyze macromolecular sodium hyaluronate into sodium hyaluronate oligosaccharide molecules; the molecular weight of the macromolecular sodium hyaluronate is not less than 1000kDa, and the molecular weight of the sodium hyaluronate oligosaccharide Not greater than 10kDa. Preferably, the temperature of the enzymatic hydrolysis is 45-55° C., and the pH is 4.8-5.2.
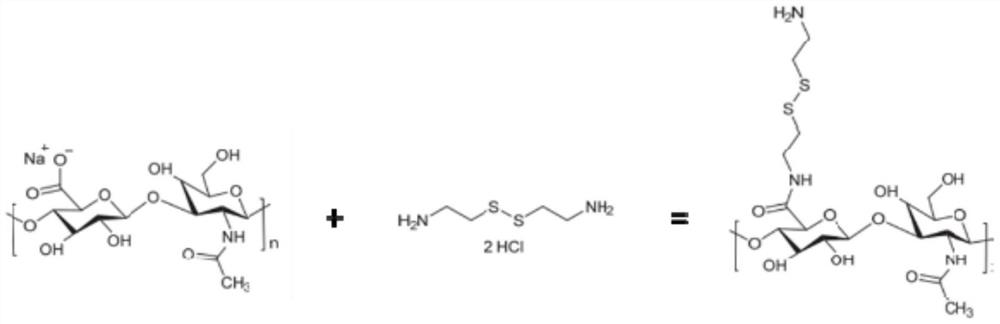
[0030] S2, condensing sodium hyaluronate oligosaccharide molecules with cystamine dihydrochloride in the presence of a coupling agent to prepare a hyaluronic acid single disulfide bond product. Preferably, the coupling agent is selected from one or both of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide.
[0031] Preferably, the step S2 includes: dissolving sodium hyaluronate oligosaccharide...
Embodiment 1
[0042] (1) 1000kDa sodium hyaluronate solution: accurately weigh 10g of 1000kDa sodium hyaluronate molecule, stir and dissolve with deionized water, and prepare a solution with a concentration of 10g / L. Store in a 4°C refrigerator for later use.
[0043] (2) Enzymolysis: remove 100ml of the prepared 1000kDa sodium hyaluronate solution, adjust the pH to 5.0, heat the temperature to 50°C with water isolation, add 100ul hyaluronidase (CAS: 37259-53) with an activity of 150000U / L -3), the enzymatic hydrolysis reaction time is 4h, and the magnetic stirrer is used to stir slightly during the reaction, and then freeze-dried.
[0044] (3) Preparation of sodium hyaluronate oligosaccharide molecular liquid: take the 1000 kDa hyaluronic acid solution after the enzymatic hydrolysis reaction, inactivate it, vibrate it with ultrasonic waves, and then place it in a refrigerator at 4°C for refrigeration. Dialyze with a modified dialysis membrane to ensure that the molecular weight is lower t...
Embodiment 2
[0050] (1) 1000kDa sodium hyaluronate solution: accurately weigh 10g of 1000kDa sodium hyaluronate molecule, stir and dissolve with deionized water, and prepare a solution with a concentration of 10g / L. Store in a 4°C refrigerator for later use.
[0051] (2) Enzymolysis: remove 100ml of the prepared 1000kDa sodium hyaluronate solution, adjust the pH to 5.0, heat the temperature to 50°C with water isolation, add 100ul hyaluronidase (CAS: 37259-53) with an activity of 150000U / L -3), the enzymatic hydrolysis reaction time is 4h, and the magnetic stirrer is used to stir slightly during the reaction, and then freeze-dried.
[0052] (3) Preparation of sodium hyaluronate oligosaccharide molecular liquid: take the 1000 kDa hyaluronic acid solution after the enzymatic hydrolysis reaction, inactivate it, vibrate it with ultrasonic waves, and then place it in a refrigerator at 4°C for refrigeration. Dialyze with a modified dialysis membrane to ensure that the molecular weight is lower t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com