MMP-9 nano antibody as well as preparation method and application thereof
A technology of MMP-9 and nano-antibody, which is applied in the field of biomedicine, can solve the problems of poor specific binding ability, weak stability and side effects, etc., and achieve the effect of improving affinity, simple and convenient preparation method, and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
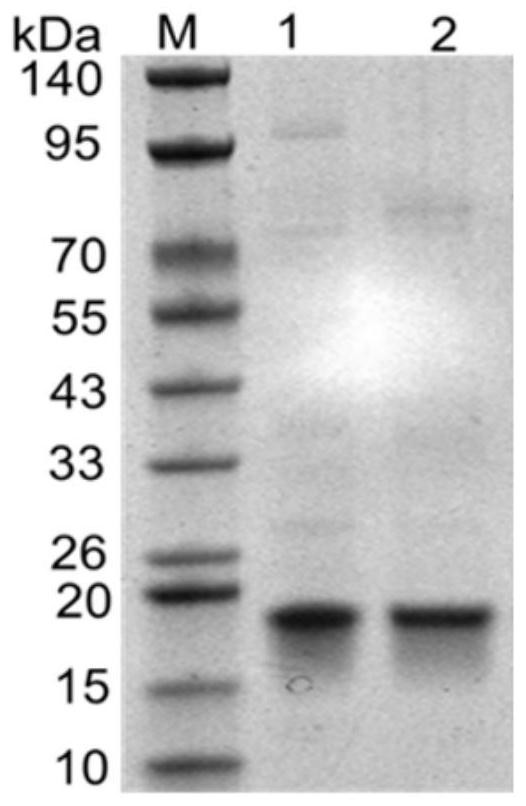
Image
Examples
preparation example Construction
[0069] The second aspect of the embodiment of the present application provides a method for preparing MMP-9 Nanobody, comprising the following steps:
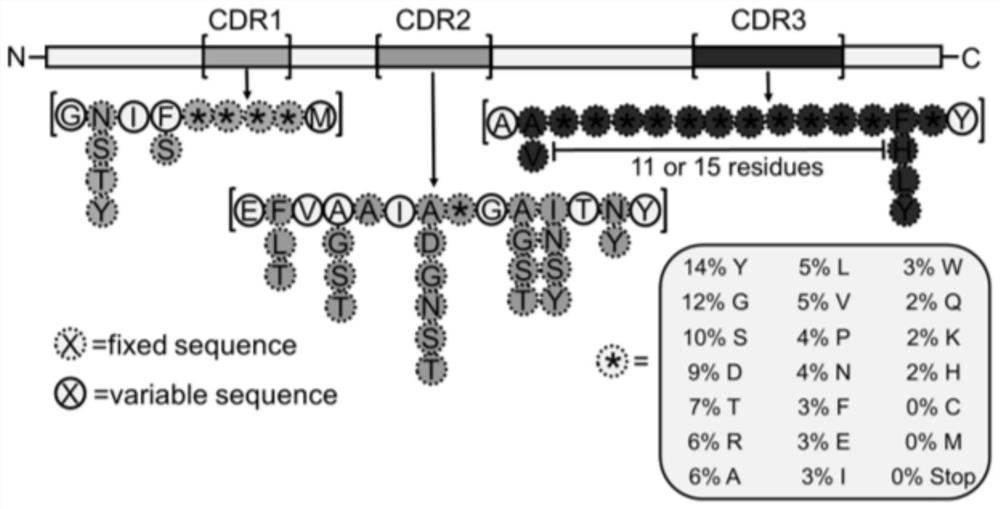
[0070] S01. Design the amino acid mutation sites of CDR1, CDR2 and CDR3 in the CDR region and synthesize the CDRs mutation primers through a large number of sequencing and comparative analysis of multiple natural libraries;
[0071] S02. Provide the synthesized Nanobody template sequence, perform codon optimization on the Nanobody template sequence and introduce a restriction enzyme cleavage site to obtain an optimized Nanobody template sequence;
[0072] S03. Insert the optimized nanobody template sequence into the phagemid carrier to construct a recombinant phagemid, perform phage display on the recombinant phagemid and extract single-stranded DNA, and use the Kunkel mutation method to pair CDRs mutation primers with single-stranded DNA to synthesize heterologous double-stranded DNA, and transformed into Escherichia coli to o...
Embodiment 1
[0111] MMP-9 nanobody and preparation method thereof
[0112] MMP-9 nanobody
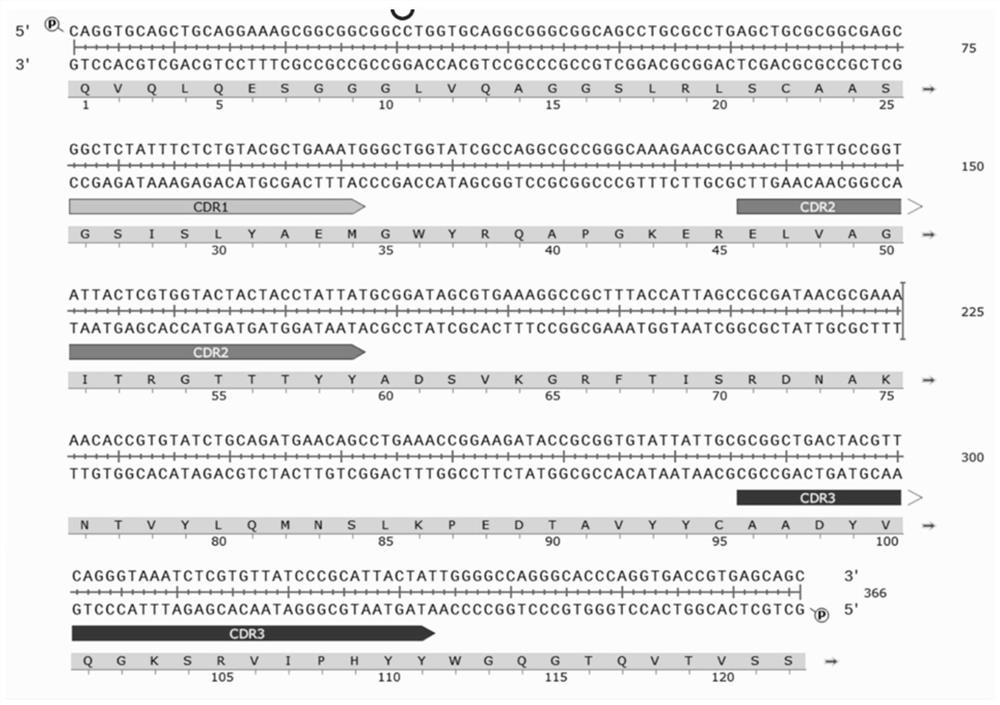
[0113] The complete amino acid sequence of the MMP-9 Nanobody G3CC is shown in SEQ.ID No.39, which is:
[0114] MKKTAIAIAVALAGFATVAQAAQVQLQESGGGLVQAGGSLRLSCAASGNIFRYRSMGWYRQAPGKERELVAGISRGTTTYYADSVKGRFTISRDNAKNTVYLQMNSLKPEDTAVYYCAVYRNQRVYGRGPVLRPLKYWGQGTQVTVSSGQAGQHHHHHHGAYPYDVPDYAS*.
[0115] Preparation method of MMP-9 nanobody
[0116] The preparation method of MMP-9 nanobody comprises the following steps:
[0117] S01. Design the amino acid mutation sites of CDR1, CDR2 and CDR3 in the CDR region and synthesize the CDRs mutation primers through a large number of sequencing comparison analysis of multiple natural libraries, wherein the mutation primers of CDR1, CDR2 and CDR3 are as follows;
[0118]
[0119] S02. Provide the synthesized Nanobody template sequence, perform codon optimization on the Nanobody template sequence and introduce a restriction endonuclease cutting site to obtain an o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com