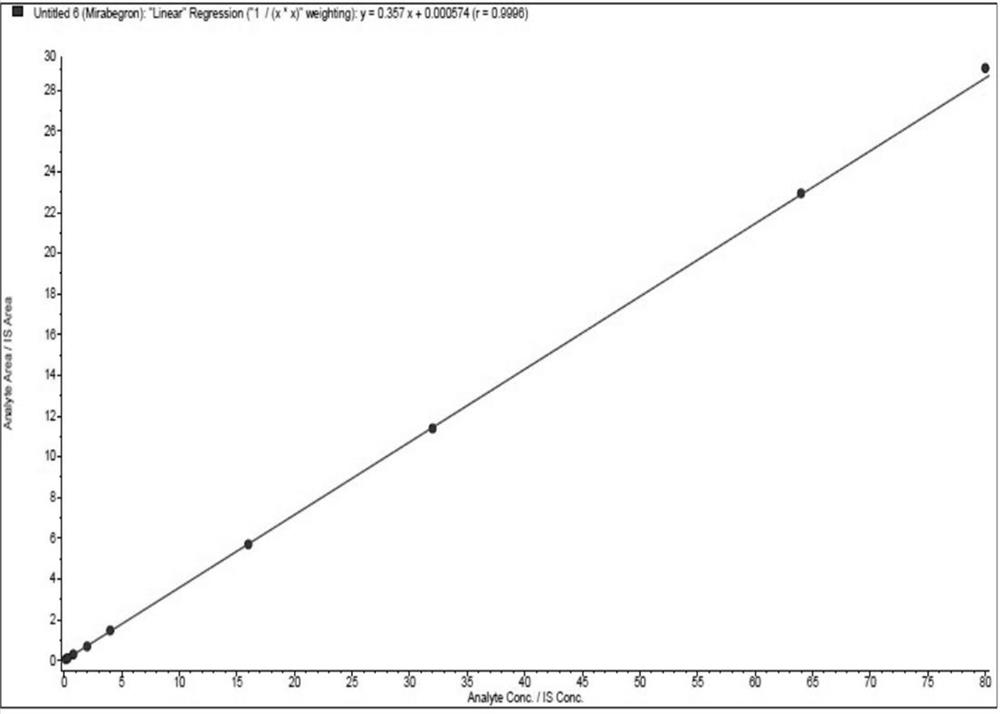
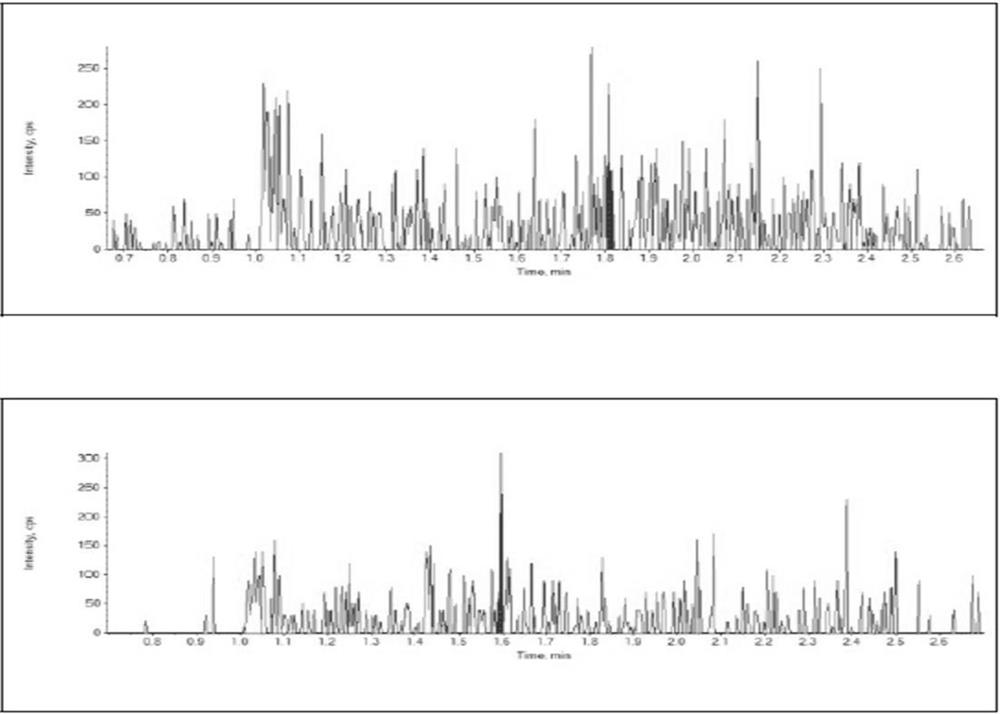
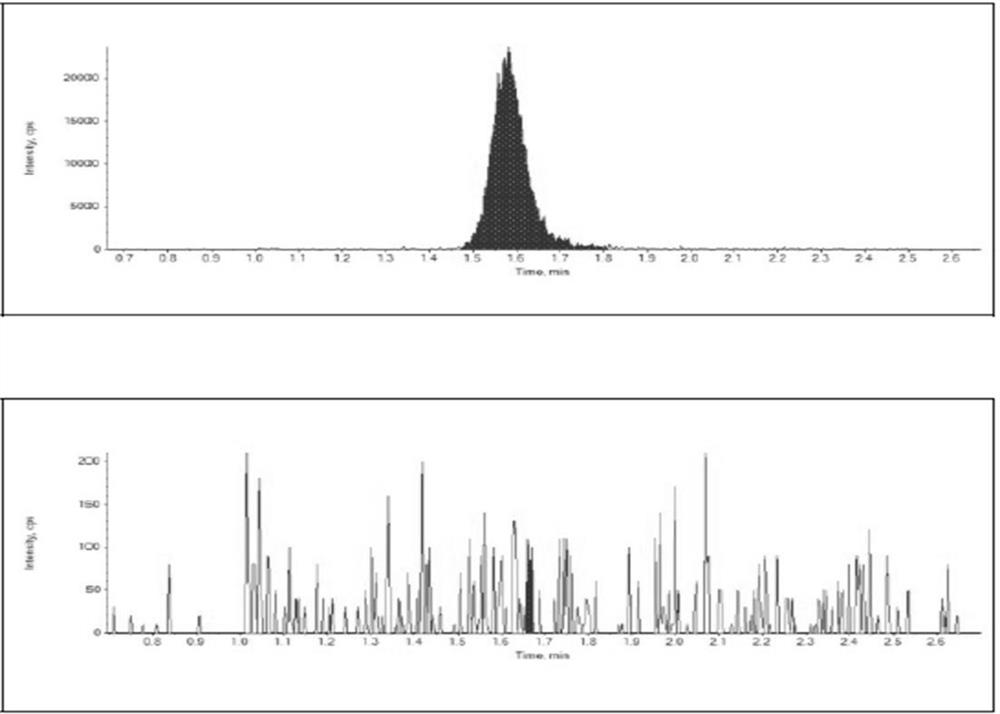
Quantitative analysis method for mirabegron in human plasma
A quantitative analysis, human plasma technology, applied in the field of biological analysis, can solve the problems of poor practicability, troublesome operation, etc., and achieve the effects of strong specificity, reduced impact, and improved detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: a kind of quantitative analysis method of mirabegron in human plasma
[0057] 1. Experimental materials and instruments
[0058] 1. Drugs and reagents: mirabegron (TLC); mirabegron-d5 (TLC); methanol (chromatographically pure, OCEANPAK); formic acid (chromatographically pure, Aladdin); ammonium formate (chromatographically pure, Aladdin); Sodium chloride (AR grade, Tianjin Damao); purified water (Wahaha).
[0059] 2. Instruments: The names and specifications of the specific instruments are shown in Table 6 below.
[0060] Table 6 Names and Specifications of Specific Instruments
[0061]
[0062] 2. Liquid quality conditions
[0063] 1. Liquid Chromatography Conditions
[0064] Chromatographic column: Synergi Hydro-RP C18 (4.0μm, 2.0*50mm);
[0065] Mobile phase: mobile phase A: (water / formic acid / 5M-AF=100 / 0.1 / 0.1), mobile phase B: (methanol / water / formic acid / 5M-AF=90 / 10 / 0.1 / 0.1);
[0066] Column oven temperature: 40°C;
[0067] Flow rate: 0.3ml / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com