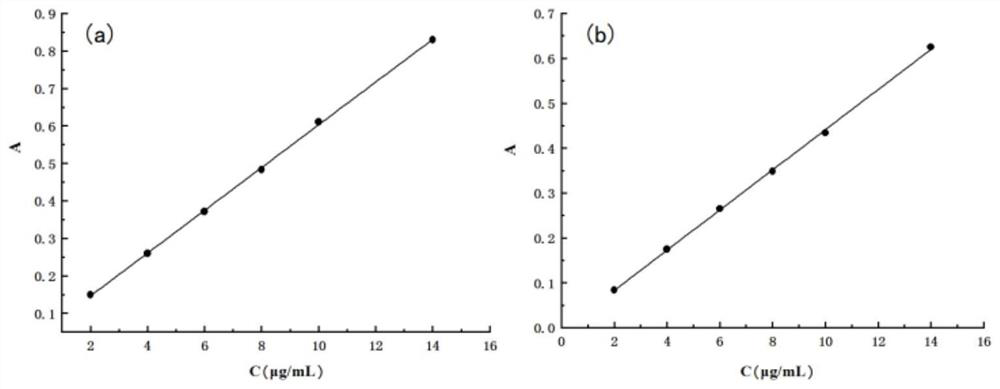
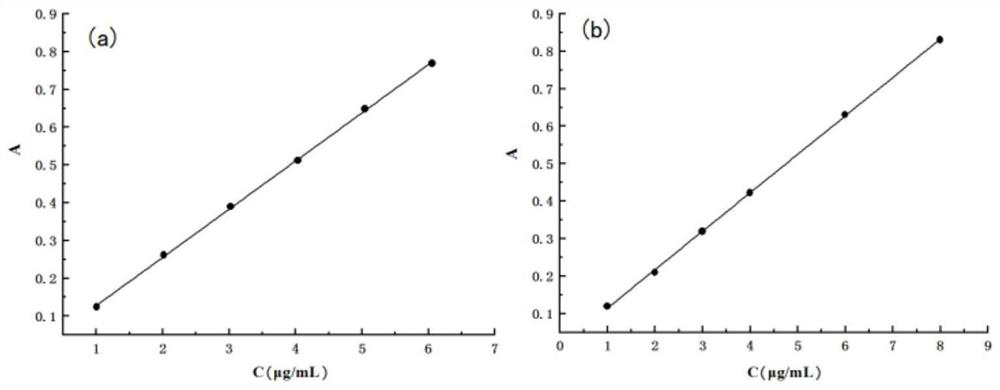
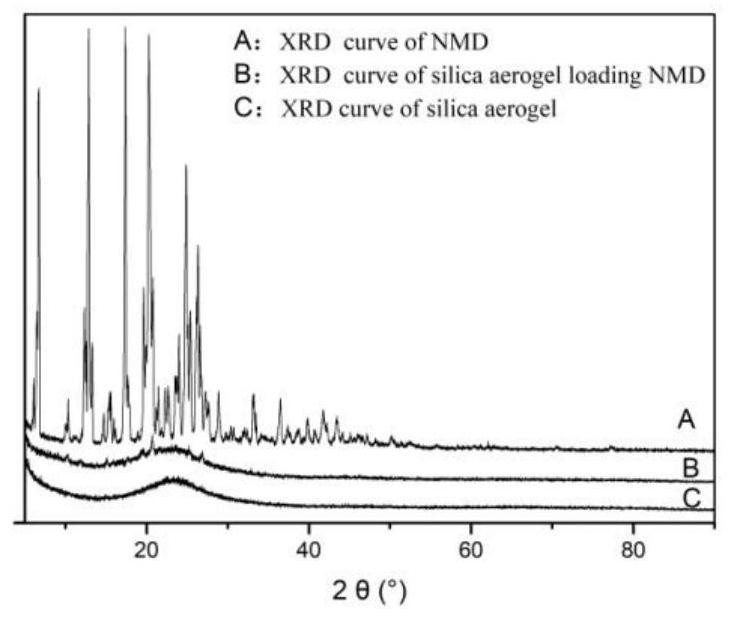
Construction and evaluation method of nano drug delivery system based on silica aerogel
A silicon aerogel, nano-drug loading technology, applied in nano-drug, nano-technology, nano-technology and other directions, to achieve the effect of good recovery, good specificity and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] A method for constructing a nanometer drug-carrying system based on silica airgel, comprising the following steps:
[0081] (1) Select insoluble drugs, and choose hydrophilic silica airgel as the carrier to carry insoluble drugs;
[0082] (2) The centrifugal impregnation method is used for drug loading, and the silicon airgel carrier is added after dissolving the drug powder in an organic solvent, and the ratio of the drug to the silicon airgel carrier is 4:1;
[0083] (3) Set drug loading parameters, mix under stirring conditions for drug loading, centrifuge at 5000r / min for 5min after drug loading, separate the precipitate, and dry the precipitate under reduced pressure to obtain sample powder.
[0084] The further optimized technical solution of this embodiment is that the hydrophilic silica airgel material is silica airgel.
[0085] The further optimized technical solution of this embodiment is that the poorly soluble drugs include BCS class II drugs. In this exam...
Embodiment 2
[0174] A kind of method for evaluating the silica airgel nano drug-carrying system that above-mentioned embodiment 1 builds, used test instrument and test material are as follows:
[0175] The experimental materials are as follows:
[0176]
[0177]
[0178] The experimental equipment is as follows:
[0179]
[0180] Specific evaluation methods for the constructed silica airgel nano-drug loading system, including morphology analysis of silica airgel, phase analysis of silica airgel, specific surface area and pore size analysis of silica airgel, silica airgel At least one of FT-IR spectroscopic analysis, thermal stability analysis of silicon aerogel, in vitro drug release determination, and gastrointestinal stability test.
[0181] The further optimized technical solution of this embodiment is that the morphology analysis of the silica airgel includes SEM analysis and TEM analysis.
[0182] Scanning electron microscopy can observe the shape characteristics and particle...
Embodiment 3
[0212] The construction and evaluation of a nano-drug loading system based on silica airgel is similar to Example 1, except that the insoluble drugs selected in step (1) are nifedipine, olanzapine, triamcinolone, naphthalene Any one of proxen, nevirapine, oxaprozin, phenazopyridine, and phenytoin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com