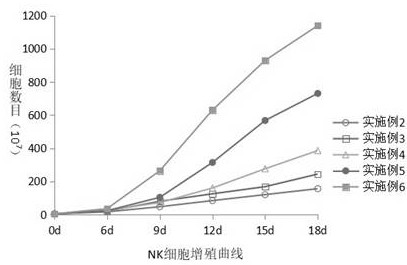
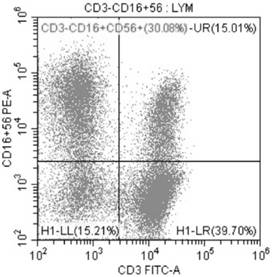
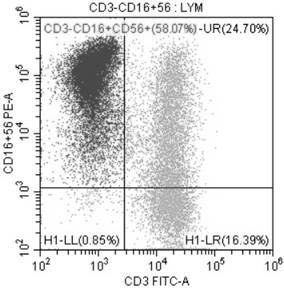
Amplification method of in-vitro NK cells and NK cells thereof
A technology of NK cells and nuclear cells, applied in the field of cell culture, can solve the problems of residual end products, different expansion potentials, and different NK cell contents, and achieve the effect of improving stability and strong killing ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]Embodiment 1: Separation and extraction of peripheral blood mononuclear cells The separation and extraction of peripheral blood mononuclear cells mainly includes the following steps:
[0042] (1) Take the peripheral blood and centrifuge at 2900r / min for 10min, take the upper plasma and inactivate it at 56°C for 30min, then centrifuge at 1500r / min for 10min, and take the supernatant as autologous plasma for later use.
[0043] (2) Use sodium chloride injection to dilute the remaining blood cell layer in step (1), and blow and mix well, take 30mL of diluted blood and slowly add it to the surface of 15mL lymphatic separation liquid, and centrifuge at 1500rpm / min for 20min; Aspirate the mononuclear cell layer into a new 50mL centrifuge tube, 15-20mL per tube, use sodium chloride injection to make up to 45mL, centrifuge at 1700rpm / min for 10min, remove the supernatant, and obtain crude mononuclear cells ;
[0044] (3) Second washing: resuspend the mononuclear cells obtained ...
Embodiment 2
[0047] A method for expanding NK cells in vitro, comprising the following steps:
[0048] (1) Preparation of magnetic microsphere suspension coupled with IL15 protein:
[0049] (S1) Mix His-tag protein purification magnetic beads with a diameter of 30 μm and made of polystyrene-nickel microspheres thoroughly and put them in a centrifuge tube for magnetic separation, discard the supernatant, and then add 10 mL of binding buffer (a mixture of 20mM sodium phosphate, 500mM sodium chloride and 5-50mM imidazole), resuspend the magnetic beads, then perform magnetic separation, remove the supernatant, and obtain pretreated His-tag protein purification magnetic beads;
[0050] (S2) Resuspend the His-tagged IL15 protein in 10 mL of binding buffer, then add it to the centrifuge tube containing the pretreated His-tag protein purification magnetic beads obtained in step (S1), and place the centrifuge tube in a vortex After oscillating for 20 seconds, place the centrifuge tube on a rotary ...
Embodiment 3
[0058] A method for expanding NK cells in vitro, comprising the following steps:
[0059] (1) Preparation of magnetic microsphere suspension coupled with IL15 and CD137L protein:
[0060] (S1) Mix His-tag protein purification magnetic beads with a diameter of 30 μm and made of polystyrene-nickel microspheres thoroughly and put them in a centrifuge tube for magnetic separation, discard the supernatant, and then add 10 mL of binding buffer (a mixture of 20mM sodium phosphate, 500mM sodium chloride and 5-50mM imidazole), resuspend the magnetic beads, then perform magnetic separation, remove the supernatant, and obtain pretreated His-tag protein purification magnetic beads;
[0061] (S2) Resuspend the His-tagged IL15 and CD137L proteins in 10 mL of binding buffer, and then add them to the centrifuge tube containing the pretreated His-tag protein purification magnetic beads obtained in step (S1). Place the centrifuge tube on a vortex shaker for 20 seconds, then place the centrifug...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com