Medical hemostatic polysaccharide dressing core
A polysaccharide and core coating technology, which is applied in the field of medical hemostatic polysaccharide core coating, can solve the problems of reduced wound healing efficiency, poor ventilation of wound dressings, weak absorption of secretions, etc. Effects of cell migration and cell proliferation, promoting wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
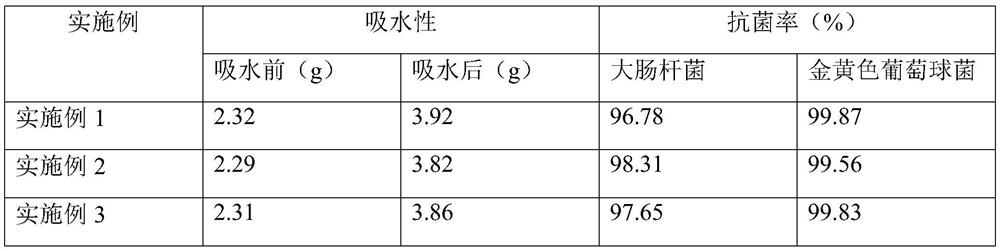
[0024] The preparation steps of the alginate dressing layer: (1) Take 8 parts of carboxymethyl chitosan, 1 part of shell hyaluronic acid chitosan, 0.1 part of antibacterial peptide, 0.5 part of sodium carboxymethyl cellulose, epidermal cells Add 0.3 parts of growth factor and 0.3 parts of collagen to 52 parts of water, mix well to obtain a functional soaking solution; (2) take 6 parts of alginate fiber non-woven fabric and immerse in the soaking solution prepared in step (1) for 6 hours, The alginate fiber non-woven fabric is taken out, freeze-dried and irradiated to obtain the alginate dressing layer.
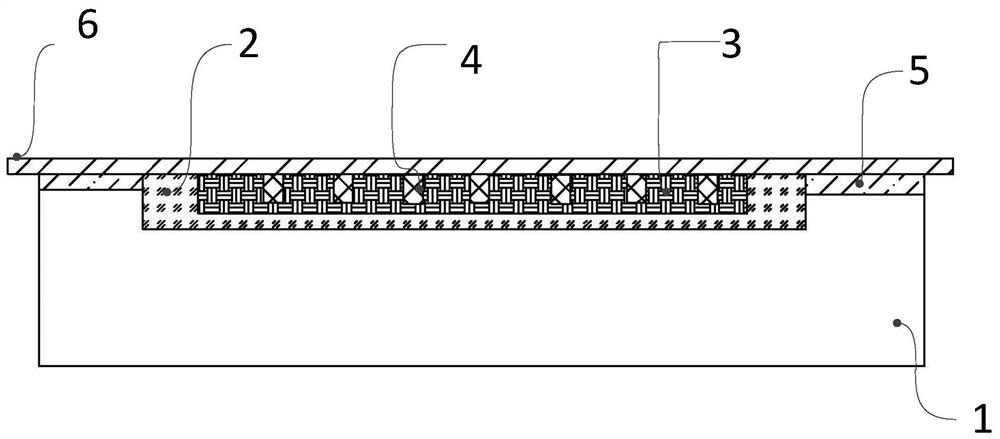
[0025] A medical hemostatic polysaccharide core, comprising a laminated composite waterproof breathable film, chitosan fiber film layer, hyaluronic acid fiber film layer, alginate dressing layer, adhesive layer, glossy surface release paper layer; the center of the waterproof breathable film is set It is a structure with an annular groove, the chitosan fiber film layer is arra...
Embodiment 2
[0026] Embodiment 2: the preparation operation steps of alginate dressing layer: (1) get 10 parts of carboxymethyl chitosan, 2 parts of shell class hyaluronic acid chitosan, 0.13 part of antimicrobial peptide, sodium carboxymethyl cellulose 3 parts 0.4 parts of epidermal growth factor and 0.3 parts of collagen were added to 60 parts of water and mixed evenly to obtain a functional soaking solution; (2) 4 parts of alginate fiber non-woven fabric were immersed in the soaking solution prepared in step (1) After 6 hours, the alginate fiber non-woven fabric was taken out, freeze-dried and irradiated to obtain the alginate dressing layer.
[0027] A medical hemostatic polysaccharide core, comprising a laminated composite waterproof breathable film, chitosan fiber film layer, hyaluronic acid fiber film layer, alginate dressing layer, adhesive layer, glossy surface release paper layer; the center of the waterproof breathable film is set It is a structure with an annular groove, the ch...
Embodiment 3
[0028] Embodiment 3: the preparation operation steps of alginate dressing layer: (1) get 11 parts of carboxymethyl chitosan, 3 parts of shell class hyaluronic acid chitosan, 0.15 part of antimicrobial peptide, sodium carboxymethyl cellulose 6 parts 0.5 parts of epidermal growth factor and 0.5 parts of collagen were added to 72 parts of water and mixed evenly to obtain a functional soaking solution; (2) Take 3 parts of alginate fiber non-woven fabrics and immerse them in the soaking solution prepared in step (1) After 6 hours, the alginate fiber non-woven fabric was taken out, freeze-dried and irradiated to obtain the alginate dressing layer.
[0029] A medical hemostatic polysaccharide core, comprising a laminated composite waterproof breathable film, chitosan fiber film layer, hyaluronic acid fiber film layer, alginate dressing layer, adhesive layer, glossy surface release paper layer; the center of the waterproof breathable film is set It is a structure with an annular groov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com