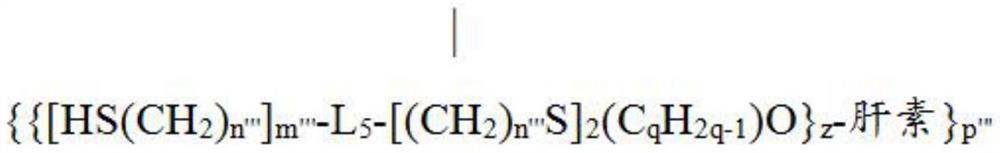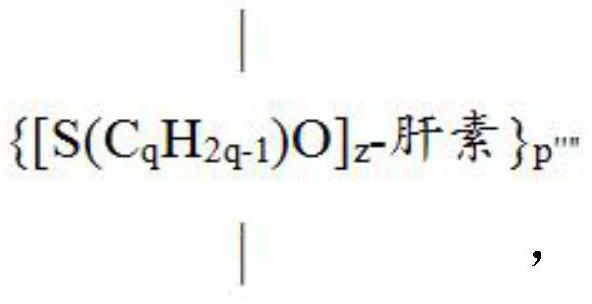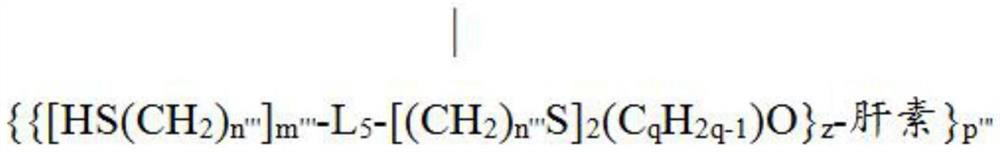Medical device comprising covalently bonded heparin coating
A covalent bonding and medical device technology, applied in medical packaging, coating, packaging, etc., can solve the problem that heparin cannot be used as an active site
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0304] Coating of heparin-TBA (40) via carbamate formation reaction (25) on PHMDI-coated polyurethane tubing (15)
[0305] To prepare the heparin-coated device (50) of the present invention, heparin-TBA (40) can be used directly in the carbamate formation reaction 25 (in acetonitrile), since the hydroxyl groups of heparin can directly interact with the PHMDI-coated tube 15 The reaction of the isocyanate group ( figure 2 ).
[0306] In a typical reaction, heparin-TBA40 (500 mg) and anhydrous acetonitrile (10 mL) were added to a 50 mL Schlenk flask in a glove box (argon protection), and a solution was obtained by shaking gently for ~5 min. The Schlenk flask was then removed from the glove box, connected to a Schlenk line (protected with nitrogen), and heated to 60°C in an oil bath.
[0307] Two freshly prepared PHMDI-coated PU tubes (prepared according to general procedure 2) were immersed in the solution and kept at 60 °C for 6 h. During the coating time of 6 h, the Schlenk...
Embodiment 2
[0310] On PHMDI or HDI coated polyurethane tubing (15), heparin-O (CHR 1 CHR 2 O) n H-TBA(60)
[0311] In addition to using heparin-TBA for direct coating onto isocyanate-coated medical devices, other modified forms of heparin-TBA can also be used (40). In one embodiment of the present invention, heparin-TBA (40 ) to modify ( image 3 ).
[0312] The ring-opening reaction of epoxides with the hydroxyl groups of heparin-TBA allows the repositioning of some of the hydroxyl groups away from the heparin carbon backbone to facilitate carbamate-forming reactions with isocyanates (25). This means that epoxy-modified heparin can be coated onto PU tubing more efficiently or more easily than heparin-TBA.
[0313] Generally, the reaction can be carried out at elevated temperature with or without a catalyst to facilitate the reaction. Examples of catalysts are triethylamine, dimethylaminopyridine (DMAP) and 1,1,3,3-tetramethylurea. The general formula of epoxide-modified heparin (...
Embodiment 3
[0321] On PHMDI-coated polyurethane tubing (15), APC cross-linked heparin-TBA (40) or APC cross-linked heparin-O (CHR 1 CHR 2 O) n H-TBA(60)
[0322] Heparin-TBA(40) or epoxy-modified heparin-O(CHR 1 CHR 2 O) n H-TBA(60) can be further modified to form intermolecular crosslinks with its respective neighboring heparin molecules. In a specific embodiment of the present invention, cross-linking can be achieved by reacting the hydroxyl group of 40 or 60 with linker 42 (i.e., adipoyl chloride (APC)) in the presence of a base (triethylamine) to obtain a cross-linked product 70 ( Figure 4 ). Alternatively, 40 or 60 can be used to react with linkers containing multiple epoxy groups to achieve intermolecular crosslinking. Intermolecular crosslinking increases the bonding density of heparin on isocyanate-coated medical devices, thereby reducing the leakage of heparin from medical devices. In this way, the lifetime of the heparin coating obtained on the device can be increased....
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com