Transaminase mutants and application thereof
A transaminase and mutant technology, applied in the field of enzyme engineering, can solve the problems of large column pressure, weak strength, continuous flow reaction, etc., and achieve the effect of increasing possibility, high space-time yield, and reducing emulsification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Mutant 50°C, pH 10, 50% DMSO tolerance test
[0080] The crude enzyme was treated at 50°C, pH 10, and DMSO concentration was 50% for 1h, then in a 10mL reaction flask, 0.1g of substrate 2 was added, and 4eq of isopropylamine hydrochloride and 0.6-1mg of PLP (5'- pyridoxal phosphate), and then add 4 mg of the enzyme after the above treatment, and stir for 16 hours at 50° C., pH 10, and DMSO concentration of 50% in an environment with constant temperature. The conversion rate of the system was detected by HPLC, and the mutant reaction data are shown in Table 5.
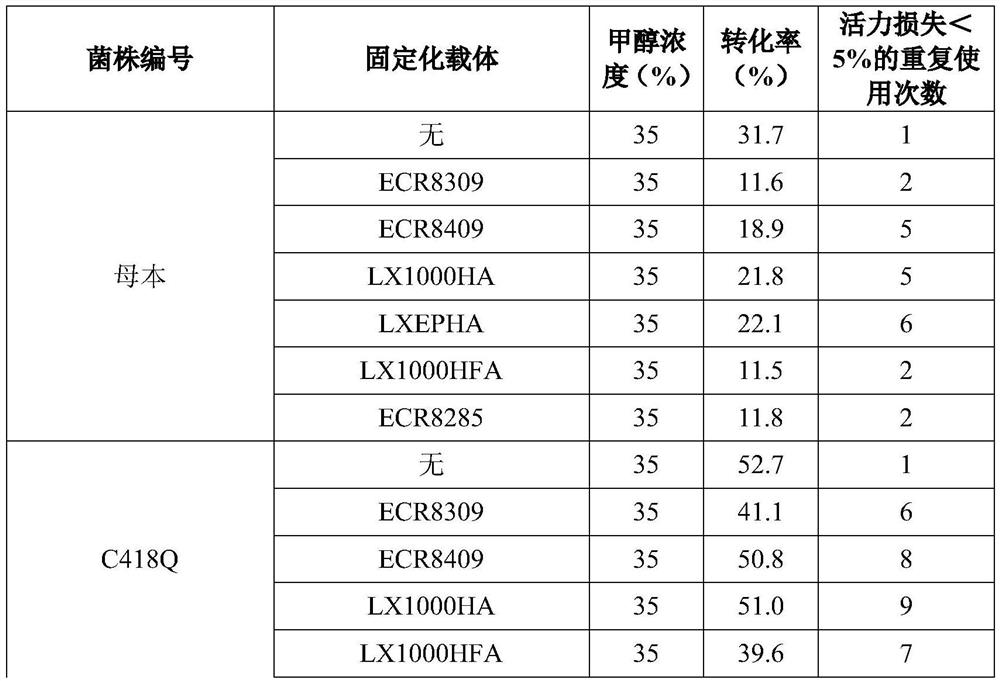
[0081] table 5:
[0082]
[0083]
Embodiment 2
[0084] Example 2: Mutant 60°C, pH 10, 60% DMSO tolerance test
[0085] The crude enzyme was treated for 1 h at 60°C, pH 10, 60% DMSO concentration environment, then in a 10mL reaction flask, 0.1g substrate 2 was added, and 4eq isopropylamine hydrochloride and 0.6mg PLP (5' - pyridoxal phosphate), and then add 5 mg of the above-mentioned enzyme, and continue to stir at 60° C., pH 10, and 60% MeOH concentration environment for 16 hours. The conversion rate of the system was detected by HPLC, and the reaction data of the mutants are shown in Table 6 below.
[0086] Table 6:
[0087] mutation site Conversion rate(%) Female parent (TA-Cv-1) 34.4 W60Y 84.3 Y168A 44.6 V379W 85.8 V379L 77.9 V379M 83.1 C418Q 69.0 C418W 75.2
Embodiment 3
[0088] Example 3: Mutant 60°C, pH 10, 70% DMSO tolerance detection
[0089] Treat the crude enzyme at 60°C, pH 10, 70% DMSO concentration for 1h, then add 0.1g of substrate 2, 4eq of isopropylamine hydrochloride and 0.6-1mg of PLP (5'- pyridoxal phosphate), and then add 10 mg of the enzyme after the above treatment, and continue stirring at 60° C., pH 10, and 70% DMSO concentration environment for 16 hours. The conversion rate of the system was detected by HPLC, and the reaction data of the mutants are shown in Table 7.
[0090] Table 7:
[0091]
[0092]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com