Sialic acid modified dexamethasone palmitate liposome as well as preparation and application thereof
A technology of dexamethasone palmitate and liposomes, which is applied in the field of medicine, can solve problems such as difficult breakthroughs, achieve low cost, improve targeting, and improve the effects of cell targeting in vivo and in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
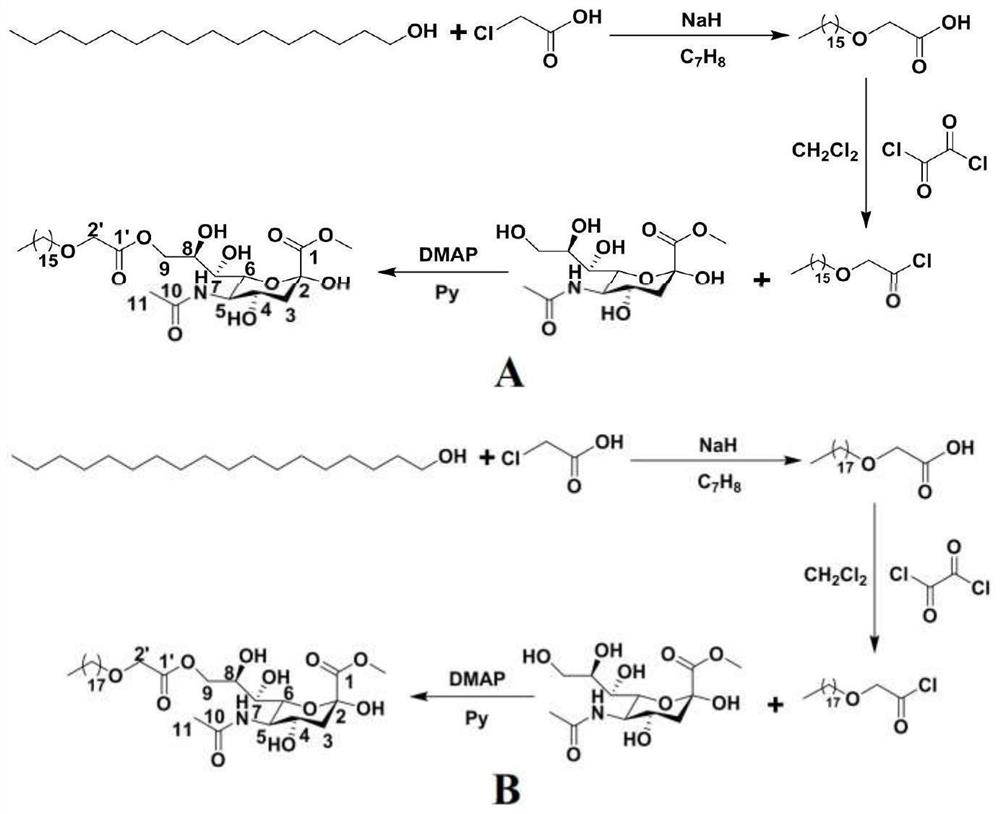
[0053] Synthesis of embodiment 1 sialic acid lipid derivatives (LYS-16 and LYS-18) (attached figure 1 A. figure 1 B)
[0054] Add 0.66g (16.5mmol) of 60% sodium hydrogen washed with petroleum ether, 10mL of anhydrous toluene, 2g (8.25mmol) of cetyl alcohol into a 100mL eggplant-shaped bottle, and add 0.94g (9.9mmol) of cetyl alcohol dropwise at 50°C for 1 hour. ) Chloroacetic acid toluene solution 5mL, dripped in 7min, and refluxed for 9 hours. Add 20mL of water, adjust the pH value to 1 with 2N hydrochloric acid, extract 3 times with 30mL ethyl acetate, wash once with 30mL saturated sodium chloride solution, anhydrous MgSO 4 Dry, filter with suction, and evaporate the filtrate to dryness to obtain white 2-(hexadecyloxy)acetic acid solid. Using the same method, white 2-(octadecyloxy)acetic acid solid was obtained from stearyl alcohol and chloroacetic acid as raw materials.
[0055] Add 1g (2.91mmol) of 2-(hexadecyloxy)acetic acid to a 100mL eggplant-shaped bottle, dissolve...
Embodiment 2
[0057] The preparation of embodiment 2 sialic acid modified dexamethasone palmitate liposomes
[0058] (1) Screening of sialic acid derivatives
[0059] Weigh HSPC, cholesterol, sialic acid derivatives and dexamethasone palmitate, add absolute ethanol with a final volume of 10% (v / v) of the preparation, stir and dissolve in a water bath at 60°C. After the solid matter is completely dissolved, open the system, continue stirring to evaporate most of the ethanol, inject 5% Glu preheated to the same temperature, and continue stirring at 60°C for 20 minutes to obtain the primary liposome. After the primary product is ultrasonically dispersed (power and time: 200W×2min+400W×6min, working for 1s and intermittent for 1s), pass through 0.80, 0.45 and 0.22μm microporous membranes in turn to obtain sialic acid-modified (unmodified) DP Liposomes.
[0060] Table 1
[0061]
[0062]
[0063] The results showed that when sialic acid-2-(hexadecyloxy)acetic acid and sialic acid-2-(oct...
example 3
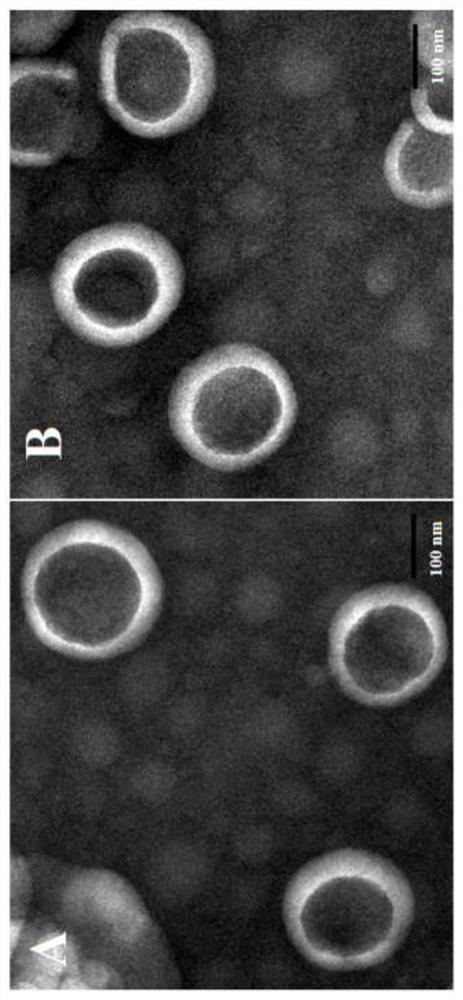
[0075] The cytostatic effect of example 3 DP liposomes (attachment image 3 )
[0076] For the separation and purification method of neutrophils, refer to patent CN201810151125. The cytostatic effect of DP liposome on neutrophils was investigated by CCK8 method.
[0077] 1. Dilute the isolated and purified peripheral blood neutrophils with RPMI 1640 culture medium to make a cell suspension, and adjust the concentration to 6×10 4 cells mL -1 .
[0078] 2. Inoculate the prepared cell suspension into a 96-well culture plate, inoculate 100 μL per well, and place at 37°C, 5% CO 2 Incubate for 1 h in the incubator. All edge wells were filled with 200 μL sterile PBS.
[0079] 3. Add each DP liposome diluted in the culture medium into a 96-well plate, add 10 μL to each well, and the final concentrations are 5, 10, 50, 100, 200 μg·mL -1 , with 3 replicate holes. At the same time, set zero wells (without cells and drugs) and control wells (with cells, without drugs), each with 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com