Synthesis method of 9-anthracene carboxylic acid
A synthesis method, the technology of anthracene carboxylic acid, is applied in the field of synthesis of dye intermediates and electronic fluorescent materials, which can solve the problems of unavailable process catalysts, unavailable 9-anthracene boronic acid, and unsuitability for industrial production, and achieve stable product quality and easy operation. Simple, less by-product effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In the reaction flask, add 100g 9-anthracene formaldehyde, 1000g isopropanol, 50g 2-methyl-2-butene, add 113.5g sodium dihydrogen phosphate and 300g aqueous solution under stirring, add dropwise 68.6g sodium chlorite and 300g aqueous solution, temperature control 20-30°C. After the dropwise addition was completed, the reaction was stirred at room temperature for 2 h. After the reaction is completed, distill under reduced pressure, distill off the solvent, adjust the pH to 2-3 with 50g of concentrated hydrochloric acid, and precipitate a large amount of solids, add 1000g of ethyl acetate to stir to dissolve the solids, filter, rinse the filter cake with 200g of ethyl acetate, and separate the liquids , the organic phase was washed twice with 300 g×2 water, and the organic phase was evaporated to obtain 74.1 g of yellow solid, yield: 69.32%.
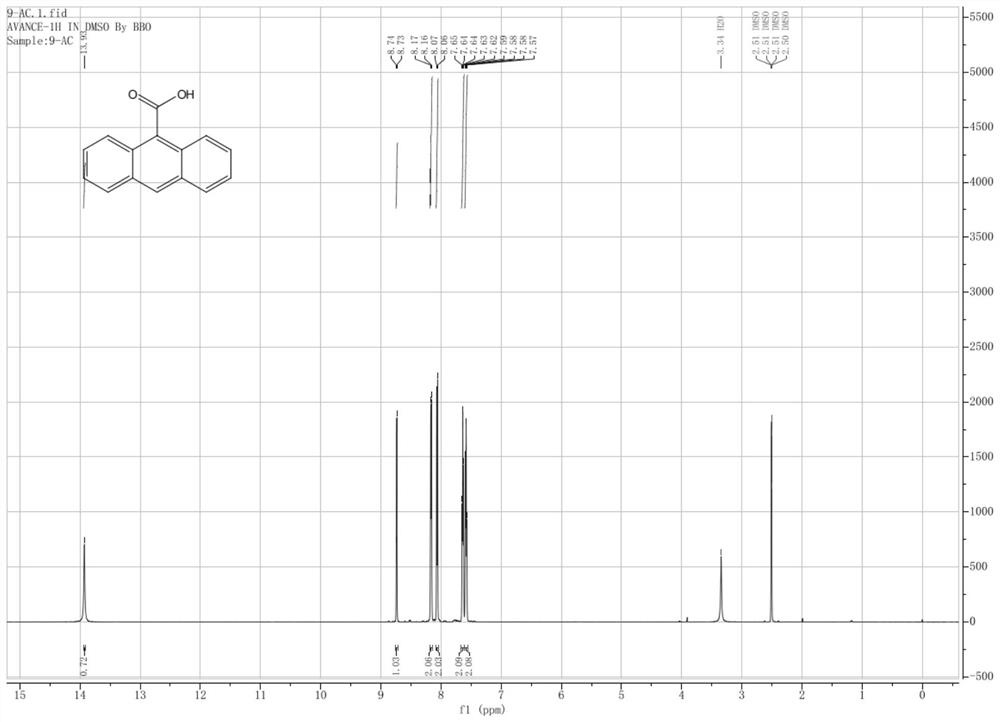
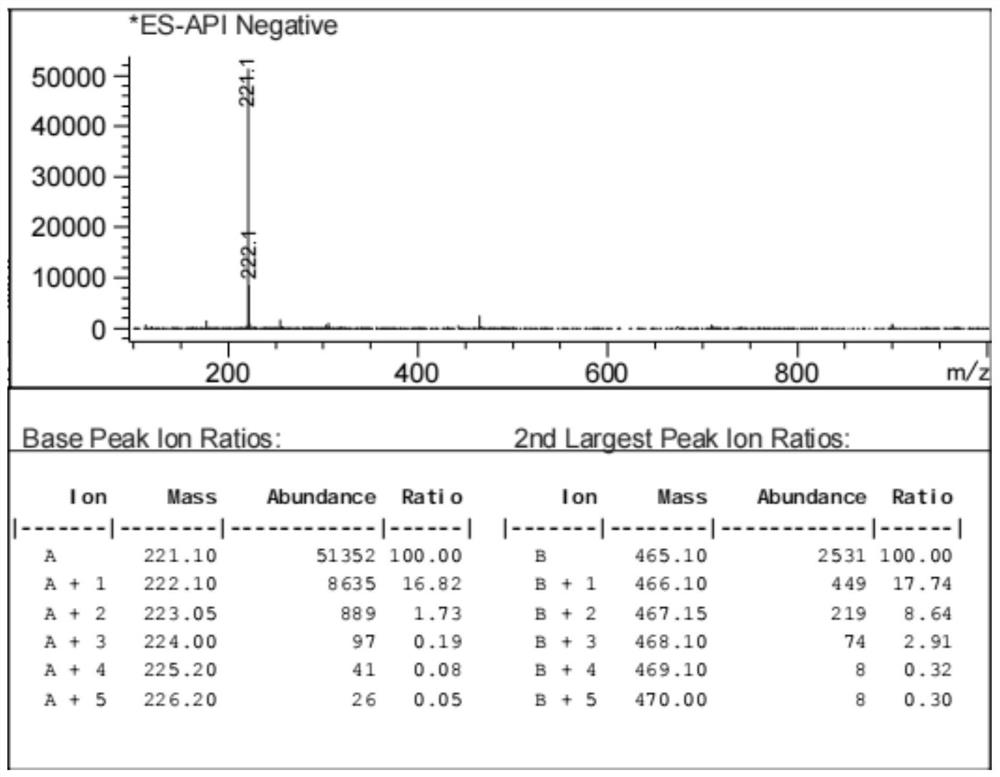
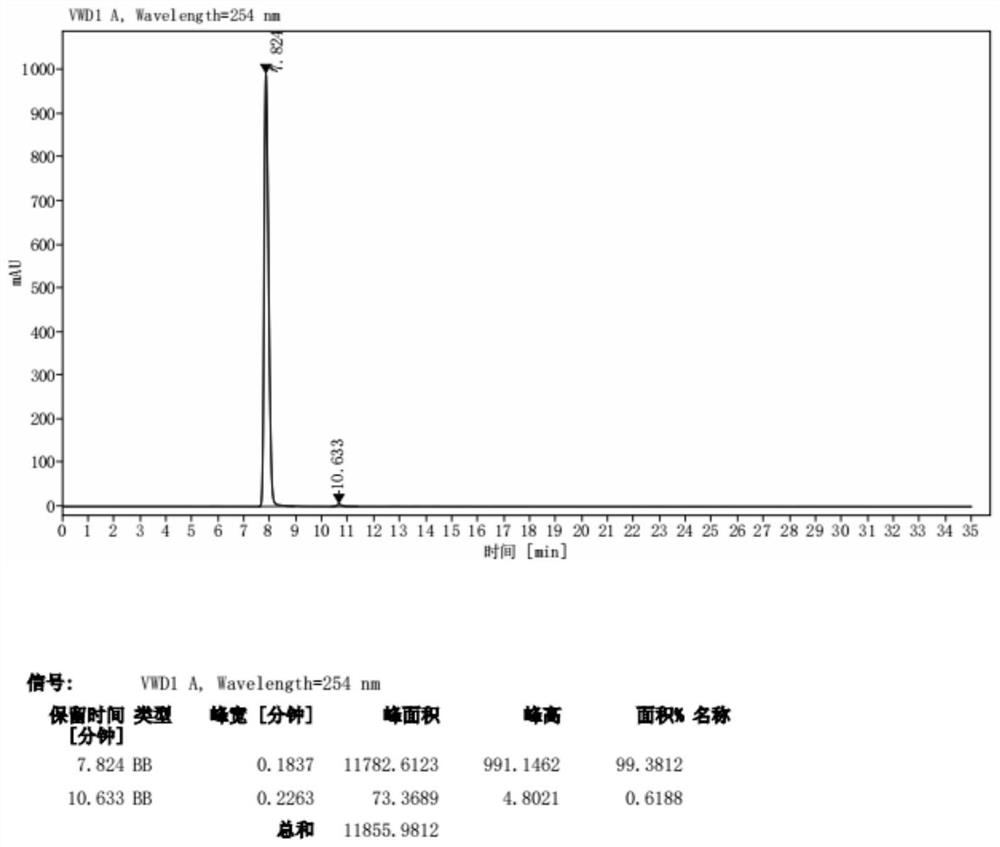
[0033] The prepared yellow solid was identified by proton nuclear magnetic spectrum, see figure 1 As shown, the results show tha...
Embodiment 2
[0037] In the reaction flask, add 100g 9-anthracene formaldehyde, 1500g isopropanol, 68g 2-methyl-2-butene, add 151g sodium dihydrogen phosphate and 300g aqueous solution under stirring, add dropwise 87g sodium chlorite and 300g aqueous solution , temperature control 20-30 ℃. After the dropwise addition, stir the reaction at room temperature for 2 hours, distill under reduced pressure, distill off the solvent, adjust the pH to 2-3 with 50 g of concentrated hydrochloric acid, and precipitate a large amount of solid, add 1000 g of ethyl acetate and stir to dissolve the solid, filter, and filter the cake with 200 g of ethyl acetate Rinse, separate liquid, wash the organic phase twice with 300 g×2 water, evaporate the organic phase to obtain 72 g of yellow solid, yield: 67.29%.
Embodiment 3
[0039] In the reaction flask, add 100g 9-anthracene formaldehyde, 1000g isopropanol, 34.02g 2-methyl-2-butene, add 151g sodium dihydrogen phosphate and 300g aqueous solution under stirring, add dropwise 39.47g sodium chlorite and 300g aqueous solution, temperature control 20-30°C. After the dropwise addition, stir the reaction at room temperature for 2 hours, distill under reduced pressure, distill off the solvent, adjust the pH to 2-3 with 50 g of concentrated hydrochloric acid, and precipitate a large amount of solid, add 1000 g of ethyl acetate and stir to dissolve the solid, filter, and filter the cake with 200 g of ethyl acetate Rinse, separate liquid, wash the organic phase twice with 300 g×2 water, evaporate the organic phase to obtain 75 g of yellow solid, yield: 69.65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com