Limited self-replication mRNA molecular system, preparation method and application
A molecular and limited technology, applied in the field of preparation and limited self-replication mRNA molecular system, can solve the problem that mRNA cannot achieve limited self-replication, and achieve the effect of avoiding cytotoxicity and achieving continuous expression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: Synthesis of the first mRNA
[0098] Step 1. Use GeneArtTM Gibson HiFi reaction (U.S. ThermoFisher, A46624) synthesized the DNA coding sequence of mutant replicase (the nucleic acid molecule encoding the first mRNA did not contain polyadenylic acid sequence), and after the synthesis was successful, the DNA encoding sequence of the mutant replicase was cloned into pcDNA3. 3 vector plasmids for industrial production.
[0099] 1.1 Mutant replicase DNA coding sequence: 5' untranslated region DNA sequence (SEQ ID NO.9), mutant replicase coding sequence (SEQ ID NO.2), 3' untranslated region DNA sequence (SEQ ID NO. 10), wherein, mutant replicase coding sequence (SEQ ID NO.2) is divided into nsP1 region fragment (SEQ ID NO.3), nsP2 region fragment (SEQ ID NO.4), nsP3 region fragment (SEQ ID NO.4) 5) Four DNA fragments of the nsP4 region fragment (SEQ ID NO. 6), all of which are modified fragments with high GC content. The four DNA fragments were ordered directly...
Embodiment 2
[0137] Example 2: Synthesis of Second mRNA
[0138] The synthesis steps of the second mRNA are similar to those of the first mRNA, including the following steps:
[0139] Step 1. Use GeneArtTM Gibson HiFi reaction (U.S. ThermoFisher, A46624) synthesizes the specifically modified DNA coding sequence of the target protein (the nucleic acid molecule encoding the second mRNA does not contain polyadenylic acid sequence);
[0140] Wherein, the specific modified target protein DNA coding sequence: 5' untranslated region DNA sequence (SEQ ID NO.9), replicase 5' end specific DNA sequence (SEQ ID NO.7), target protein DNA coding sequence ( Please refer to Table 6), replicase 3' end specific DNA sequence (SEQ ID NO. 8), 3' untranslated region DNA sequence (SEQ ID NO. 10).
[0141] Step 2, adding the poly-(a) tail of mRNA to the specifically modified target protein DNA coding sequence by PCR to obtain the DNA synthesis template of the second mRNA;
[0142] Step three, in vitro transcr...
Embodiment 3
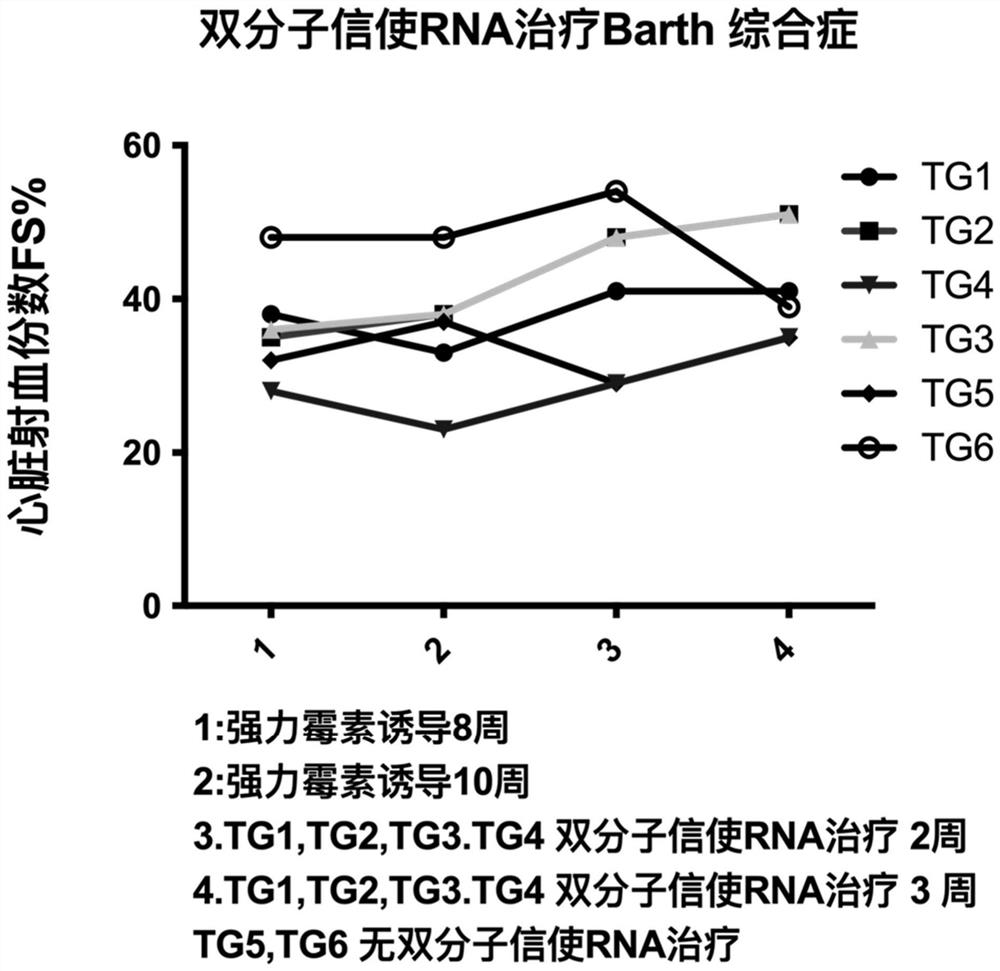
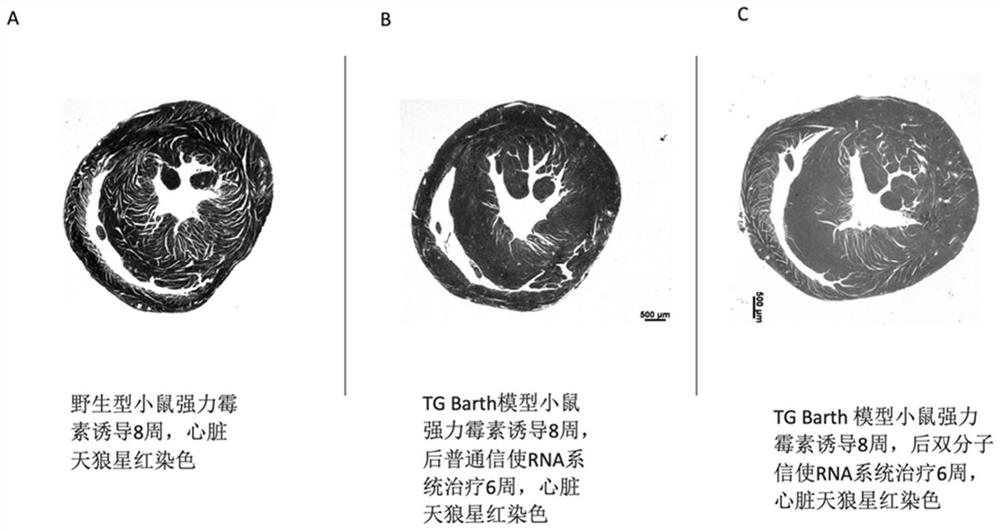
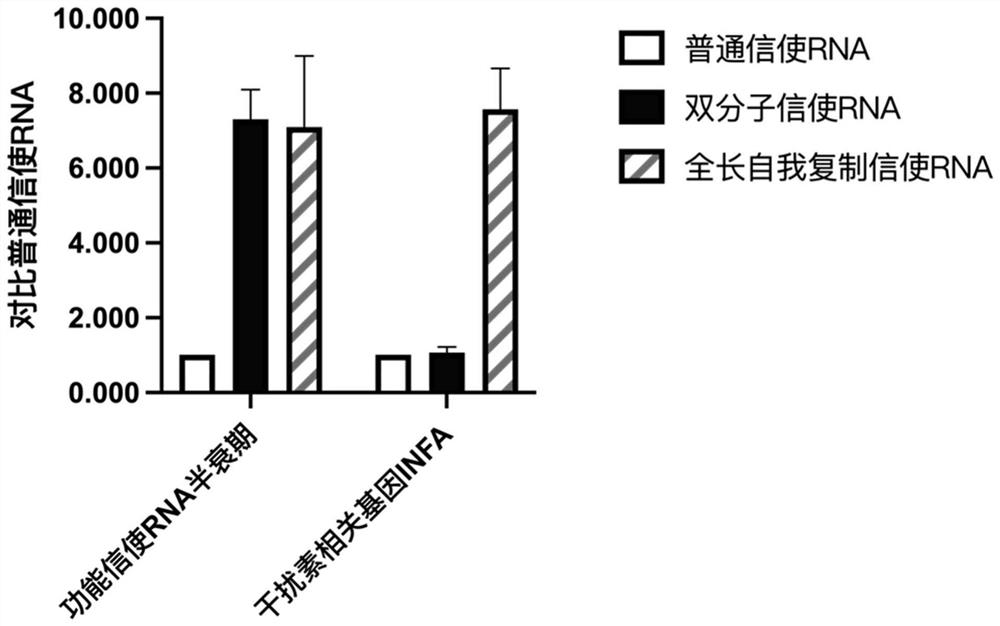
[0149] This embodiment provides a pharmaceutical composition, multiple molecular messenger RNA and a delivery carrier, wherein the multiple molecular messenger RNA includes the first mRNA prepared in Example 1 and the second mRNA-1 prepared in Example 2, and the delivery carrier is protamine protein. In this example, the target protein is Taffazin protein.
[0150] Example 3 Application-Therapeutic Experiment of Mouse Barth Syndrome Model
[0151] 3.1 Mouse Barth syndrome model and induction:
[0152] Doxycycline was introduced into the mouse genome to induce Knock down of Taffazin protein to establish a mouse model of Barth syndrome. The DNA was determined by PCR analysis for genotyping. Primers:
[0153] 5' CCATGGAATTCGAACGCTGACGTC 3' (SEQ ID NO. 45);
[0154] 3' TATGGGCTATGAACTAATGACCC 5' (SEQ ID NO. 46);
[0155] In this case, only males were used, and doxycycline was placed in the drinking water of mice at a concentration of 2 mg / L, which also contained 10% sucrose....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com