Application of Vina-ginsenoside R18 in preparation of anti-dengue virus pharmaceutical preparation
A pharmaceutical preparation, dengue virus technology, applied in the field of anti-virus, to achieve the effect of inhibiting replication, strong anti-dengue virus activity, and good medicinal prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
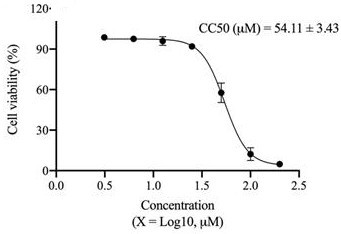
[0036] Example 1 Toxicity of Vina-ginsenoside R18 to BHK-21 cells
[0037] Test method BHK-21 cells in the logarithmic growth phase were taken in 1×10 5 Cells / mL were inoculated in 96-well plates, 100 μL per well, and the control group and drug groups with different concentrations were set up. The cytotoxicity was detected by MTT method 4 days later.
[0038] figure 1 The test results of Vina-ginsenoside R18 showed that the half inhibitory concentration of Vina-ginsenoside R18 on BHK-21 cells was 54.11 μM, and it was basically non-toxic to cells at a concentration of 25 μM.
Embodiment 2
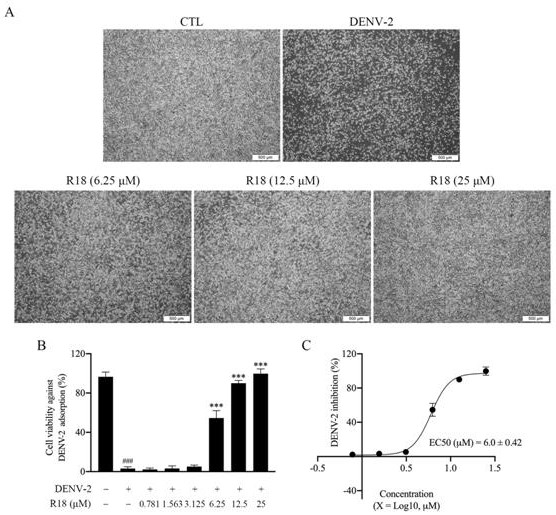
[0039] Example 2 Vina-ginsenoside R18 blocks the adsorption of type 2 dengue virus to host cells
[0040] Test method BHK-21 cells in the logarithmic growth phase were taken in 1×10 5 Cells / mL were seeded in a 96-well plate with 100 μL per well. The model group was infected with 200PFU of DENV-2, and the drug group was given 200PFU of DENV-2 and vina-ginsenoside R18 (6.25, 12.5, 25μM) at the same time. After incubation at 4°C for 1 hour, the virus and drug solution were discarded, and 2% maintenance solution was added. , placed in 37 ℃, 5% (v / v) CO 2 continue to grow in the incubator. After 4 days, the blocking effect of the drug on the adsorption of type 2 dengue virus to the host cells was determined by observing the cell lesions and death.
[0041] figure 2 The experimental results showed that Vina-ginsenoside R18 blocked the adsorption of dengue virus type 2 to host cells in a dose-dependent manner.
Embodiment 3
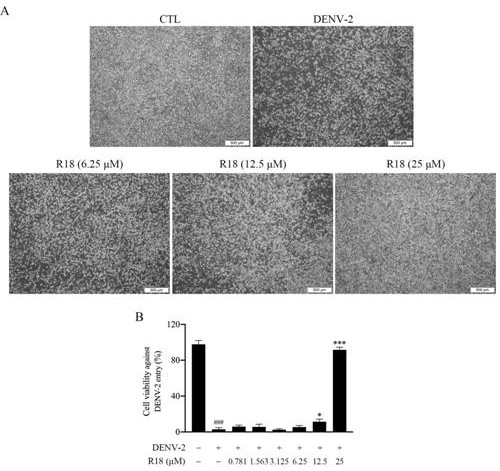
[0042] Example 3 Vina-ginsenoside R18 blocks type 2 dengue virus from entering host cells
[0043] Test method BHK-21 cells in the logarithmic growth phase were taken in 1×10 5 Cells / mL were seeded in a 96-well plate with 100 μL per well. The model group and the drug group were respectively infected with 200PFU of DENV-2, cultured at 4°C for 1 hour, discarded the virus, and the drug group was given vina-ginsenoside R18 (6.25, 12.5, 25μM), placed in 37°C, 5% (v / v )CO 2 Incubate for 1 h in an incubator. Afterwards, the drug solution was discarded, 2% maintenance solution was added, and the culture was continued in an incubator at 37°C. After 4 days, the blocking effect of the drug on the entry of type 2 dengue virus into the host cells was determined by observing the lesions and death of the cells.
[0044] image 3 The test results show that Vina-ginsenoside R18 can effectively block type 2 dengue virus from entering host cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com