Application of MxA and SAA in preparation of diagnostic reagent for discriminating children viral influenza
A technology for viral influenza and diagnostic reagents, applied in disease diagnosis, biological testing, material testing, etc., can solve problems such as short half-life of type I IFNs and no diagnostic efficacy, eliminate false positive results, and improve diagnostic sensitivity. Effects with high specificity, specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
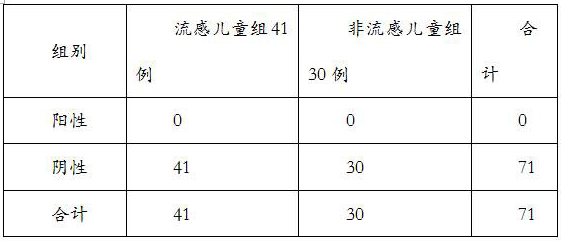
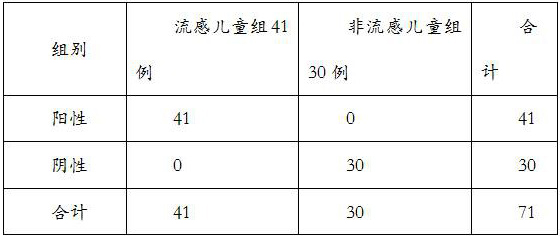
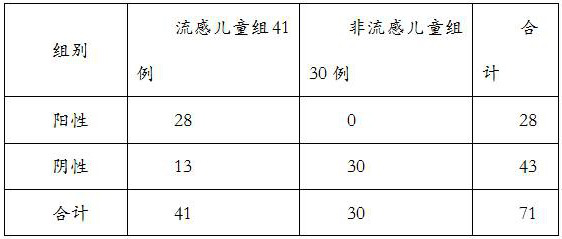
[0025] In the present invention, the application of a kind of MxA and SAA or its binding antibody or fragment in the preparation of a diagnostic reagent for screening viral influenza and non-influenza in children, said diagnostic reagent detects the content of MxA and SAA in individual samples and compares with the normal level In contrast, childhood viral influenza was diagnosed when MxA and SAA levels in individual samples exceeded normal levels.
[0026] A product for diagnosing viral influenza in a child individual who has symptoms of influenza. The diagnostic product contains a diagnostic reagent, which contains a binding antibody or binding antibody or fragment of MxA and SAA at the same time, and binds to MxA and SAA in the individual sample through the binding antibody or fragment to detect in the individual sample The MxA and SAA contents were used for diagnosis.
[0027] An application, method and product of MxA and SAA or its binding antibody or fragment in the pre...
Embodiment 2
[0038] In the present invention, a preparation method of a novel MxA and SAA duplex colloidal gold rapid diagnostic kit includes the purification of MxA, the preparation of MxA monoclonal antibody and the development of MxA / SAA, MxA, SAA and CRP rapid detection kit.
[0039] 1. Purification of MxA
[0040] Purify the MxA and MXB mixture extracted from lymphocytes by ion-exchange chromatography according to the laboratory's operating procedures. We used Pharmacia MonoQ5 / 50 ion-exchange columns from Amersham Biosciences, and the eluent gradient was 0.15 to 0.3mol / L NaCl and 50 mmol / L sodium acetate. Purified MxA was further confirmed by tandem mass spectrometry and western-blot verification.
[0041] 2. Preparation of MxA monoclonal antibody
[0042] For antigen pretreatment, take an appropriate amount of antigen and mix it with an equal volume of Freund's complete adjuvant, and use a syringe to suck and emulsify into a water-in-oil state to immunize mice;
[0043] Immunization...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com