Combined medicine composition for treating megaloblastic liver cancer and application thereof in preparation of medicine for treating megaloblastic liver cancer
A composition and a bulk-type technology, applied in the field of medicine, can solve the problems of large resection range, liver insufficiency, difficult operation, etc., and achieve the effects of prolonging the survival period, relieving the disease, and benefiting the patient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The embodiment of the application provides the combined treatment effect and safety evaluation of case 1:
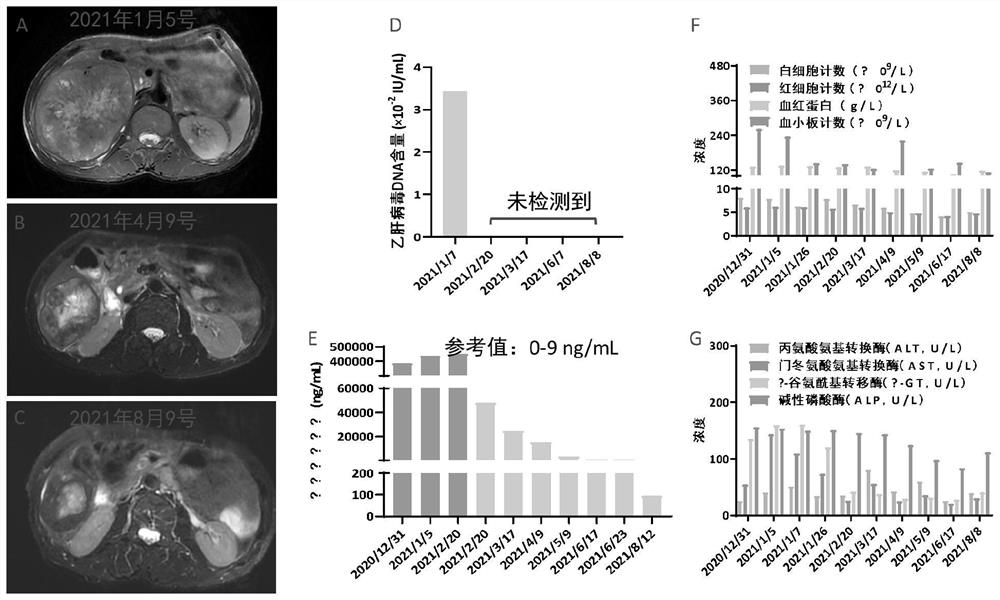
[0042] see figure 1 , figure 1 The results before and after treatment of case 1 provided in the embodiment of the present application.
[0043] The MR results on January 5, 2021 showed a huge oval mass in the right lobe of the liver at S5 / S6, about 12.4×9.8×11.6cm in size, with regular edges and clear borders. T1WI showed slightly low signal, and T2WI showed mixed slightly high signal , DWI showed high signal intensity, irregular necrosis area was seen in the center of the lesion, and the enhancement scan showed obvious heterogeneous enhancement in the arterial phase, and slow and continuous enhancement in the portal vein and delayed phase. Signal, the larger one is about 2.0×1.5×1.1cm. After enhancement, the arterial phase is obviously enhanced, the portal venous phase is rapidly reduced, the delayed phase is low signal, and the DWI is obviously high signal. T...
Embodiment 2
[0046] The embodiment of the application provides the combined treatment effect and safety evaluation of case 2:
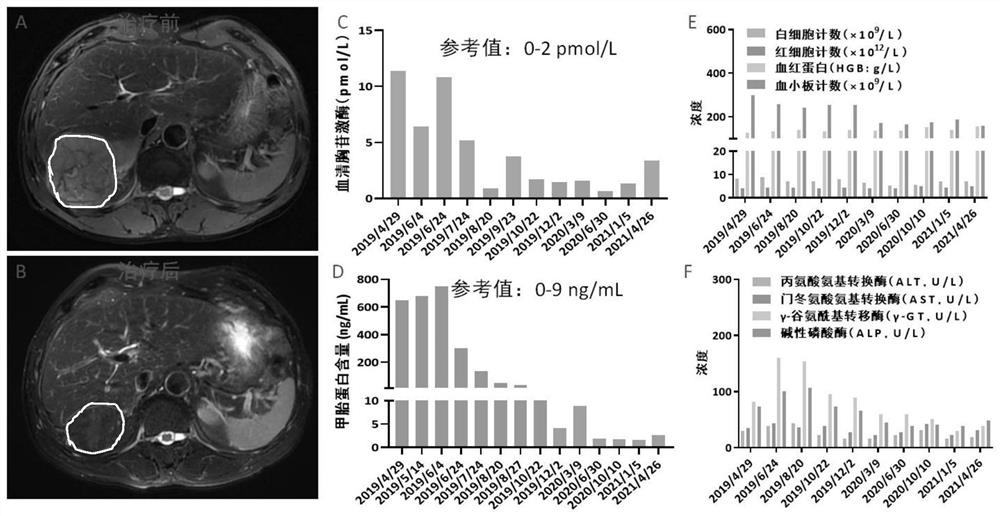
[0047] see figure 2 , figure 2 The results before and after treatment of case 2 provided in the embodiment of the present application.
[0048] The MR results on April 30, 2019 showed a tumor in the right lobe of the liver with a size of 9.4cm×8.3cm×10.7cm ( figure 2 A); On May 10, 2019, he received a tumor embolization therapy, and then gave the patient combined drugs for the treatment of massive liver cancer, receiving thymusfasin (1.6mg, once a day during hospitalization, subcutaneous injection) combined with vitamin C (100mL normal saline + vitamin C injection 3000mg, once a day during hospitalization, intravenous infusion, once a day during hospitalization), anti-PD-1 antibody (200mg / time, once every three weeks) and apatinib ( One piece each time, once a day) and S-1 (one piece each time, twice a day, taking 14 days and stopping for 7 days, 21 days as ...
Embodiment 3
[0051] The embodiment of the application provides the combined treatment effect and safety evaluation of case 3:
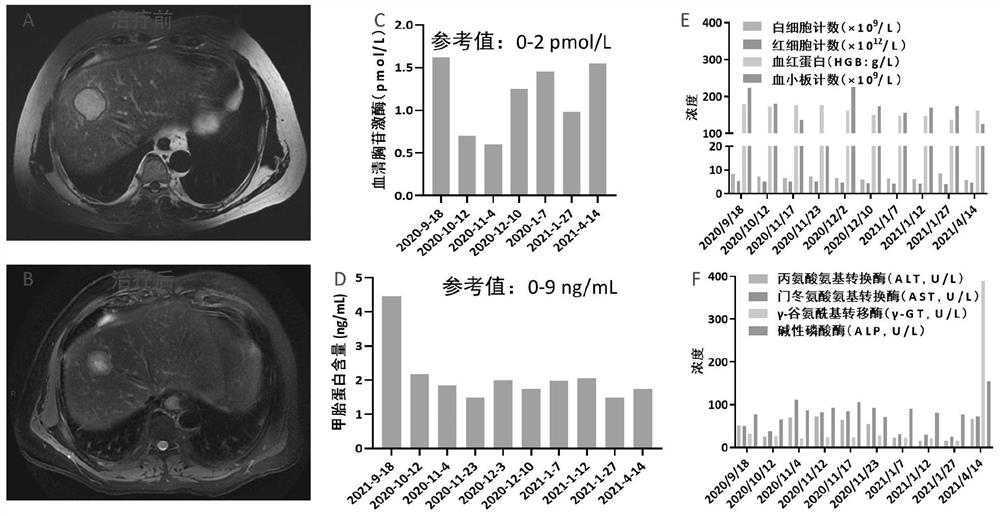
[0052] see image 3 , image 3 The results before and after treatment of case 3 provided in the embodiment of the present application.
[0053] The MR results on September 20, 2020 showed that there was a huge irregular mass in the S4 / 8 segment of the liver, with a size of about 4.7×4.5×4.2cm, with irregular edges and unclear boundaries. T1WI showed slightly low signal, and T2WI showed mixed Slightly hyperintensity, DWI showed hyperintensity, significantly heterogeneous enhancement in arterial phase of enhanced scan, portal vein decreased rapidly, and slightly hypointensity in delayed phase; no abnormality was found in portal vein and hepatic vein ( image 3 A). The patient was given combined drugs for the treatment of massive liver cancer, after thymofasin (1.6mg, once a day during hospitalization, subcutaneously injected) combined with vitamin C (100mL normal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com