Synthesis method of snake venom-like tripeptide
A synthetic method and snake venom technology, applied in the field of synthesis of snake venom-like tripeptides, can solve the problems of difficult purification, low total yield, low purification yield, etc., achieve short synthesis process route, overcome stability problems, and purify The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
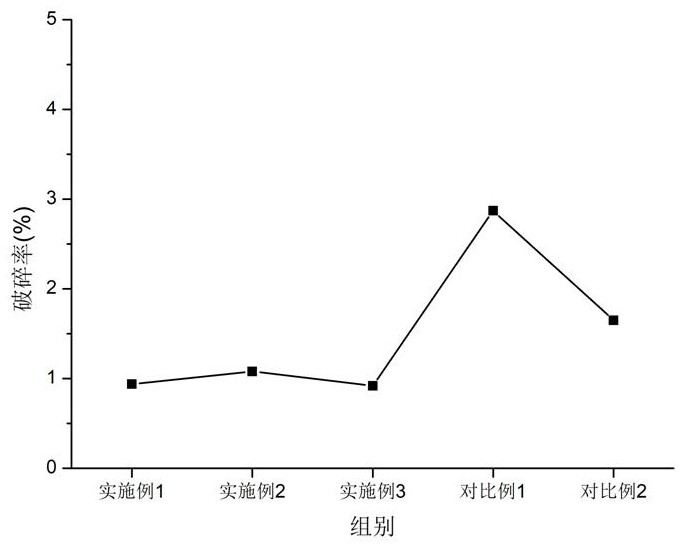
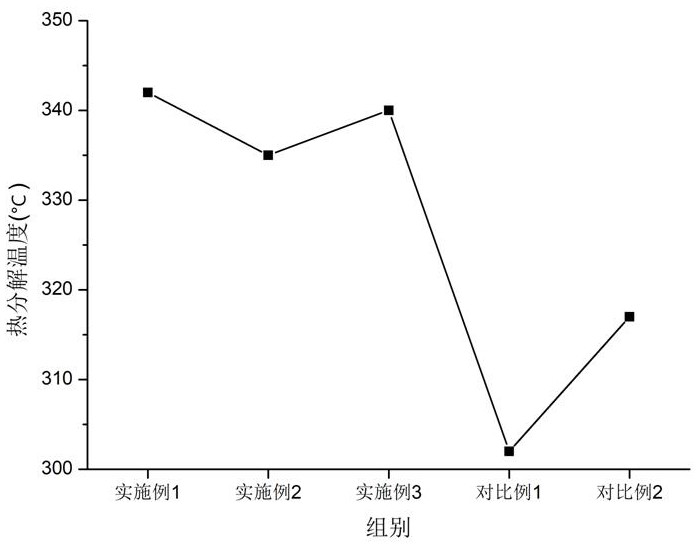
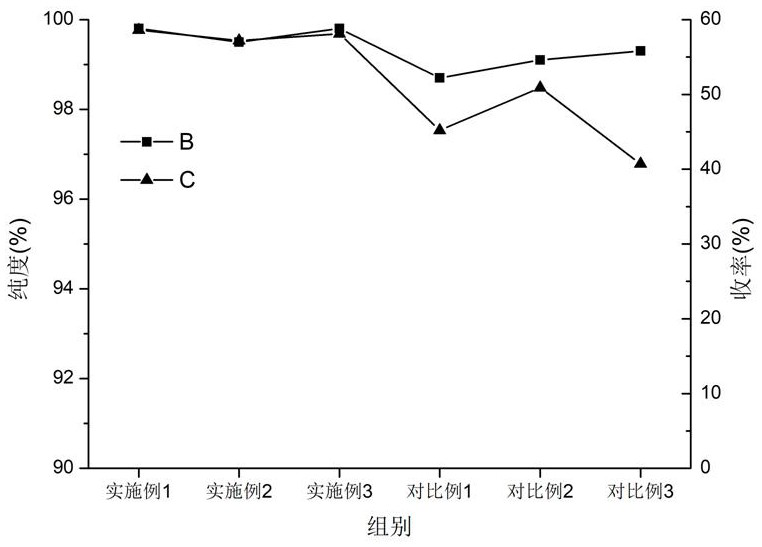
Examples
Embodiment 1
[0063] This embodiment provides a synthetic method for a snake-like venom tripeptide, the synthetic route is as follows figure 1 As shown, the method includes:
[0064] Preparation of S1, H-Dab(Boc)-NHBzl:
[0065] In 600mL tetrahydrofuran, add 44gFmoc-Dab(Boc)-OH, 11.8g benzylamine, 14.2gNMM, stir at room temperature for 5min, add 235gEDCI, stir for 24h, add water to the product to precipitate a solid, wash with water, dissolve with dichloromethane, Separate the layers, dry the organic layer, filter, remove dichloromethane by rotary evaporation, add the obtained compound to 400 mL of tetrahydrofuran solution containing 15 vol% piperidine, stir at room temperature for 30 min, concentrate by rotary evaporation, add diethyl ether to precipitate, filter, and use Washed twice with ether and dried to obtain 27.6g of H-Dab(Boc)-NHBzl;
[0066] Preparation of S2, Boc-β-Ala-Pro-OH:
[0067] In 500mL tetrahydrofuran, add 18.9g Boc-β-Ala-OH, 16.6gH-Pro-OMe HCl, 1.65gHOBT, 10gNMM, sti...
Embodiment 2
[0082] This example provides another synthesis method of snake venom-like tripeptide, which differs from Example 1 only in that during the preparation of the modified PGMA-TCA chromatographic packing, the addition amount of anophylline is 6 g.
[0083] This example also provides an anti-wrinkle cosmetic, which differs from Example 1 only in that the snake venom-like tripeptide obtained in Example 1 is used instead of the snake-like venom tripeptide obtained in Example 1.
Embodiment 3
[0085] This example provides another synthesis method of snake venom-like tripeptide, which differs from Example 1 only in that during the preparation of the modified PGMA-TCA chromatographic packing, the addition amount of anophylline is 10 g.
[0086] This example also provides an anti-wrinkle cosmetic, which differs from Example 1 only in that the snake venom-like tripeptide obtained in Example 1 is used instead of the snake-like venom tripeptide obtained in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com