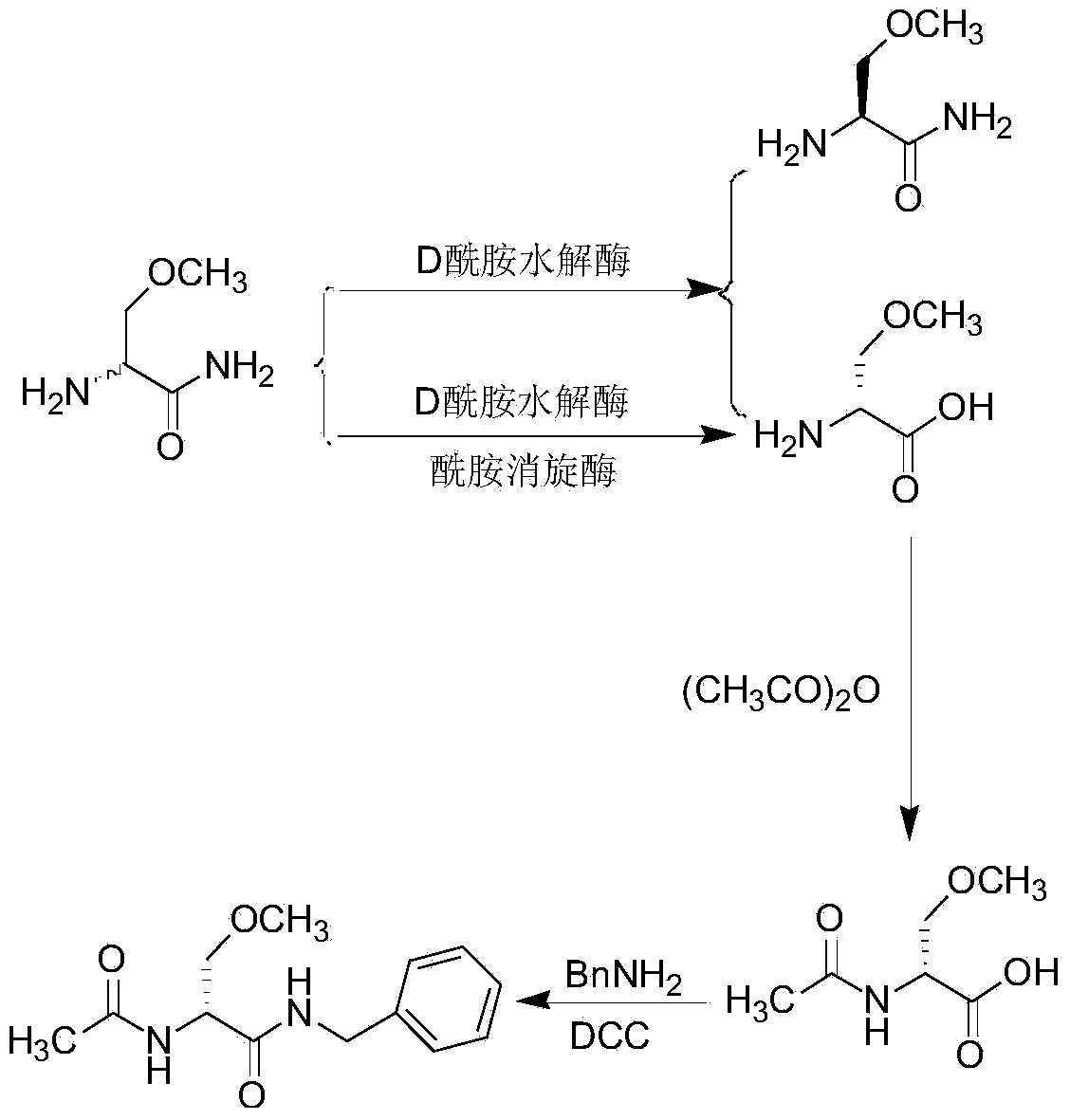
Method for preparing lacosamide by virtue of chemoenzymatic method
A chemical enzymatic method, lacosamide technology, applied in biochemical equipment and methods, microorganism-based methods, microorganisms, etc., can solve problems such as unsuitable industrial production, unsuitable industrial production, product racemization, etc., and achieve good economic benefits. and social benefits, avoid amino protection and deprotection steps, reduce the effect of environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment one: single enzyme conversion reaction
[0028] Centrifuge 1000mL of Bacillus pallidus CICC10363 fermentation broth to obtain 20g of wet bacteria, add it to 1000mL of transformation liquid, the transformation liquid contains 60g of racemic 2-amino-3-methoxypropionamide (mass concentration 6%), 1.0g / L Tween-80, pH11, enzymatic reaction at 55°C for 5h. After the reaction, the transformation solution was centrifuged at 4000r / min for 15 minutes to remove the bacterial cells, the supernatant was adjusted to pH 5.0 with 6mol / L hydrochloric acid, added 5 grams of activated carbon, stirred and heated to 70°C for decolorization, and suction filtered; the decolorization solution was concentrated in vacuum to precipitate out , cooled and crystallized, vacuum filtered, rinsed with pure water, stirred with 80% ethanol, and dried to obtain (R)-2-amino-3-methoxypropionic acid 22.1g, (R)-2-amino The conversion rate of -3-methoxypropionamide is about 80%, ee=98.1%.
Embodiment 2
[0029] Embodiment two: single enzyme conversion reaction
[0030] Centrifuge 1000mL of the fermentation broth of Bacillus pallidum NCCB10026 to obtain 19g of wet bacteria, and add it to 1000mL of transformation liquid, which contains 60g of racemic 2-amino-3-methoxypropionamide (6% mass concentration), 0.05g / L CTAB, pH 6, enzymatic reaction at 25°C for 1h. After the reaction, the transformation solution was centrifuged at 4000r / min for 15 minutes to remove the bacterial cells, the supernatant was adjusted to pH 5.0 with 6mol / L hydrochloric acid, added 5 grams of activated carbon, stirred and heated to 70°C for decolorization, and suction filtered; the decolorization solution was concentrated in vacuum to precipitate out , cooled and crystallized, vacuum filtered, rinsed with pure water, stirred with 80% ethanol, and dried to obtain (R)-2-amino-3-methoxypropionic acid 13.8g, (R)-2-amino The conversion rate of -3-methoxypropionamide is about 50%, ee=97.7%.
Embodiment 3
[0031] Embodiment three: single enzyme conversion reaction
[0032] Centrifuge 1000mL of Bacillus pallidus CICC10363 fermentation broth to obtain 22g of wet bacteria, add it to 1000mL of transformation liquid, the transformation liquid contains 60g of racemic 2-amino-3-methoxypropionamide (mass concentration 6%), 0.2g / L OP, pH9, enzymatic reaction at 45°C for 4.5h. After the reaction, the transformation solution was centrifuged at 4000r / min for 15 minutes to remove the bacterial cells, the supernatant was adjusted to pH 5.0 with 6mol / L hydrochloric acid, added 5 grams of activated carbon, stirred and heated to 70°C for decolorization, and suction filtered; the decolorization solution was concentrated in vacuum to precipitate out , cooled and crystallized, vacuum filtered, rinsed with pure water, stirred with 80% ethanol, and dried to obtain 26.3g of (R)-2-amino-3-methoxypropionic acid, (R)-2-amino - The conversion rate of 3-methoxypropionamide is about 95%, ee=98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com