Preparation method of insulin crystals and products thereof
A technology of insulin and insulin aspart, applied in the field of protein crystals, can solve the problems of hexahedral crystallization of insulin, etc., and achieve the effects of wide crystallization temperature range, easy industrial production, and shortening of freeze-drying time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0107] The preparation method of insulin crystals of the present invention may comprise the following steps: 1) mixing insulin, citric acid, phenolic substances, zinc substances, water and optional organic solvents to obtain a crystallization solution; 2) adjusting the pH using a pH regulator 1) The pH value of the crystallization solution in the medium, crystallization, and insulin crystallization.
[0108] In the preparation method of the present invention, insulin, citric acid, phenolic substances and zinc substances in the crystallization solution may have a certain dosage ratio relationship.
[0109] In one embodiment, the molar ratio among insulin, citric acid, phenolic substance and zinc substance may be 1:(127.3-1272.9):(3.1-123.0):(4.3-85.6).
[0110] In a preferred embodiment, the molar ratio among insulin, citric acid, phenolic substance and zinc substance may be 1:(127.3-1272.9):(3.1-123.0):(10.0-85.6).
[0111] In a preferred embodiment, the molar ratio among ins...
Embodiment 1
[0264] Embodiment 1 (comparative example)
[0265] The crystallization of insulin aspart is prepared with reference to the crystallization method of recombinant insulin glargine in CN106117345B.
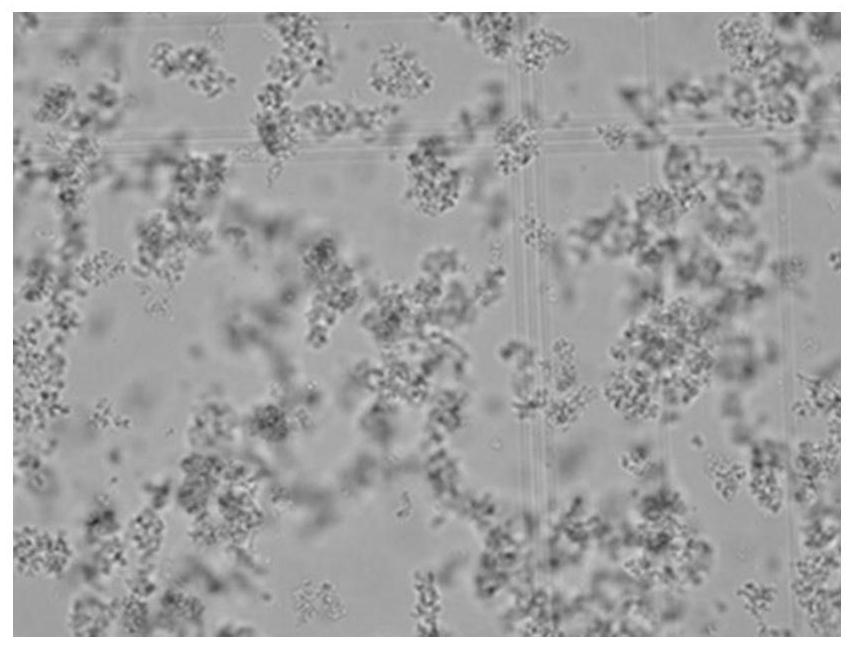
[0266] The 250mL crystallization liquid contains about 2.53g / L recombinant insulin aspart, about 0.5g / 100mL citric acid, about 0.05g / 100mL zinc chloride and about 0.1g / 100mL solid phenol, pH=5.0, wherein: The molar ratio between insulin aspart, citric acid, phenol, and zinc chloride is about 1:60.3:24.5:8.6. The specific operation is as follows: Weigh 0.63g of recombinant insulin aspart and dissolve it in 125mL of water to make recombinant aspart Insulin solution; 0.249g of phenol, 0.126g of zinc chloride and 1.25g of citric acid were added thereto, and the volume was adjusted to 250mL by adding water to prepare a crystallization solution. The crystallization solution was stirred at room temperature at a low speed, the pH value was adjusted to 5.0 with sodium hydroxide, and stirred ...
Embodiment 2
[0269] Experiment A:
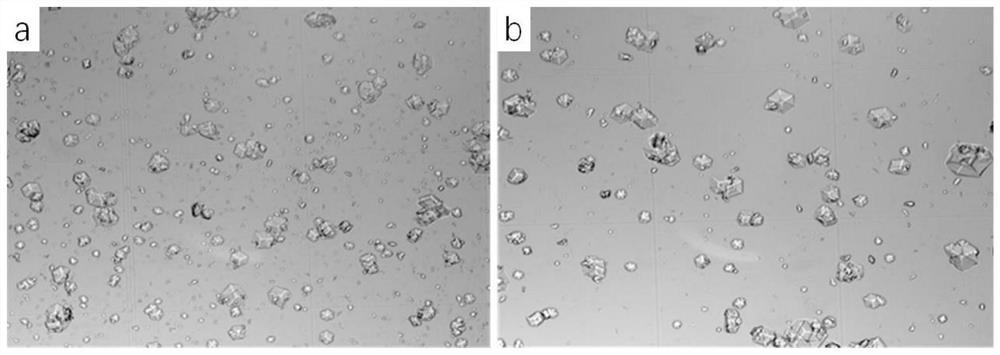
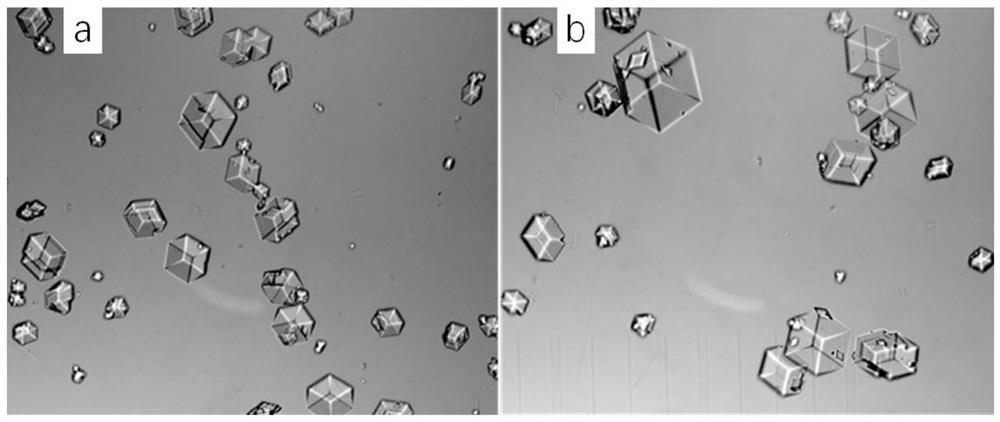
[0270] The 40mL crystallization solution contains about 0.2M citric acid, about 5g / L recombinant insulin aspart, about 0.2g / 100mL solid phenol, about the same weight of recombinant aspart zinc chloride, about 0.1v / v% ethanol, pH=6.0, wherein: the molar ratio among recombinant insulin aspart, citric acid, phenol and zinc chloride is about 1:255.0:24.6:42.8.
[0271] Experiment B:
[0272] The crystallization solution contains about 5v / v% ethanol, and the other components are the same as in Experiment A.
[0273] The specific operation is as follows: dissolve recombinant insulin aspart in water to prepare a 10g / L recombinant insulin aspart solution, and dissolve solid phenol in water to prepare a 30g / L phenol solution for use; take two 50mL triangular flasks, each Add 20mL of recombinant insulin aspart solution to each flask, then add 1.68g of citric acid, 2.65mL of phenol solution and 200mg of zinc chloride to each flask, and then add 40μL and 2mL to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com