Characteristic chromatogram of Dingkundan as well as construction method and application of characteristic chromatogram
A feature map and construction method technology, applied in the field of construction of traditional Chinese medicine feature map, can solve the problems of inaccurate quality monitoring, lack of inclusion, etc., achieve the effect of convenient operation, increase the consistency rate, and reduce the possibility of manual processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] The construction of embodiment 1 Dingkun Dan (big honey pill) HPLC characteristic collection of illustrative plates
[0085] 1.1 Raw material
[0086] Dingkun Dan (Big Honey Pill): batch numbers are 190101, 190132, 190133, 190341, 190165, 190412, 190725, 190828, 190829, 191013, 191038, 191039, 191222; each pill weighs 10.8g, Inner Mongolia Jingxin Pharmaceutical Co., Ltd. company.
[0087] 1.2 Equipment and model
[0088] High performance liquid chromatography: Agilent1260Infinity, DAD detector, Agilent Technologies Co., Ltd.;
[0089] Chromatographic column: Inertsil ODS-3C18 (4.6×250mm, 5μm), Japan GLSciences Company;
[0090] Analytical balance: METTLERTOLEDOAL204 (1 / 10,000), Mettler-Toledo Instruments (Shanghai) Co., Ltd.;
[0091] Analytical balance: METTLERTOLEDOXS105 (1 / 100,000), Mettler Toledo Instruments (Shanghai) Co., Ltd.;
[0092] Electronic balance: UTP-313, d=0.01g, Shanghai Huachao Electric Co., Ltd.;
[0093] Pharmacopoeia sieve: No. 3 sieve, Zhej...
Embodiment 2
[0121] Example 2 Dingkundan Control Characteristic Spectrum and Similarity Evaluation
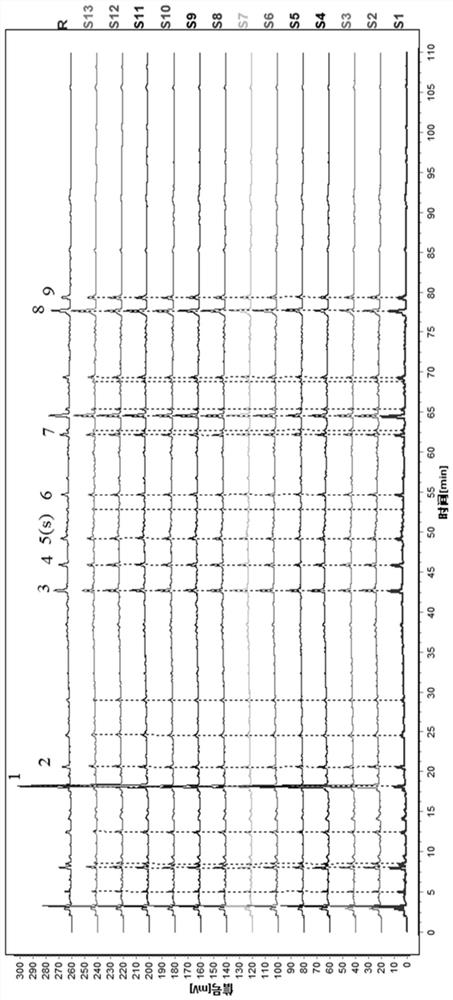
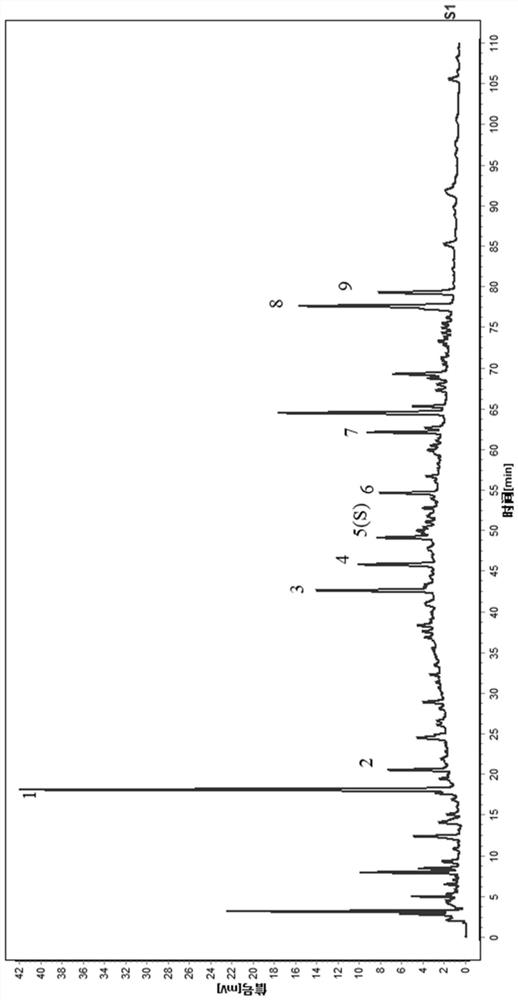
[0122] Get Dingkundan (big honey pill), operate by the preparation and assay method of need testing solution in embodiment 1, continuous sample injection 6 times, based on the chromatogram of above-mentioned 13 batches of samples, use the pharmacopoeia committee to recommend "Chinese medicine chromatographic feature map similarity evaluation system" (2008 edition) carries out data processing and obtains common pattern feature map, is Dingkundan contrast feature map R (such as figure 2 As shown), the retention time of each main chromatographic peak of the 6 injection samples is basically the same, and it is determined that the 9 peaks separated by this detection condition are their common chromatographic peaks.
[0123] The analysis was carried out according to the established Dingkundan characteristic map analysis method, and the similarity was calculated according to the similarity evalua...
Embodiment 3
[0126] Embodiment 3 methodological verification
[0127] The HPLC chromatographic conditions determined in Example 1 were investigated for repeatability and stability.
[0128] (1) Repeatability
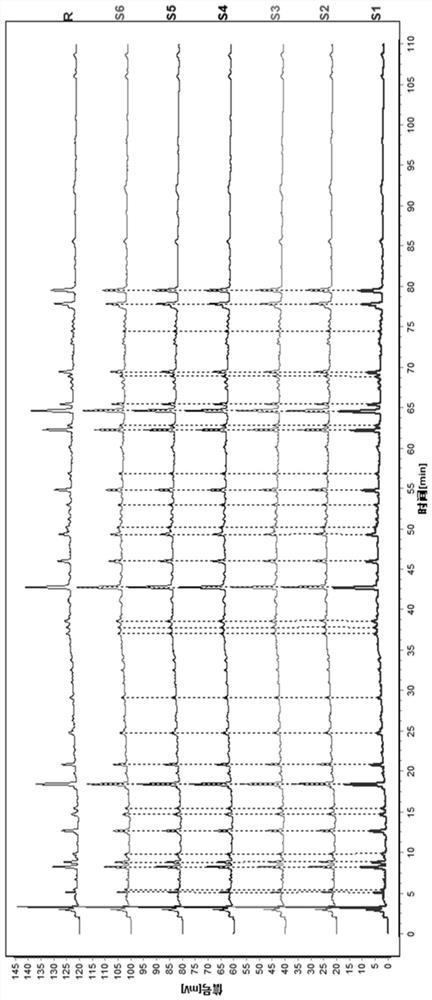
[0129] Get Dingkun Dan (big honey pill), operate under the preparation and assay method of need testing solution according to embodiment 1, calculate its similarity by Chinese medicine chromatographic fingerprint spectrum similarity evaluation system, the results are shown in Figure 5 , Image 6 .
[0130] Figure 5-6 Middle: S1 is repeatability 1, S2 is repeatability 2, S3 is repeatability 3, S4 is repeatability 4, S5 is repeatability 5, S6 is repeatability 6, and R is the control fingerprint.
[0131] The results showed that the similarity was greater than 0.95, indicating that the method had good repeatability.
[0132] (2) Stability
[0133] Get Dingkun Dan (big honey pill), operate under the preparation and assay method of need testing solution according to embodiment 1, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com