Crystal form of heterocyclic compound as well as preparation method and application of crystal form
A compound and crystal form technology, applied in the field of medicinal chemistry, can solve problems such as unrelated compound crystal forms, and achieve strong economic value, convenient storage, and high reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176] Embodiment 1: the preparation of formula A compound
[0177]
[0178] Step 1 (S)-2-((2-(4-bromo-2,6-difluorophenyl)-7-chloroimidazo[1,2-a]pyridin-3-yl)methyl)morpholine - Preparation of 4-tert-butyl carboxylate
[0179]
[0180] In a 100mL round bottom flask, (S)-2-ethynylmorpholine-4-carboxylic acid tert-butyl ester (3.1g, 1.0eq, intermediate 1-4), 4-bromo-2,6-difluorobenzene Formaldehyde (2.76g, 1.0eq, compound 172-1), 4-chloropyridin-2-amine (1.61g, 1.0eq, compound 172-2), CuCl (0.37g, 0.3eq), Cu(OTf) 2(1.36g, 0.3eq), isopropanol (50mL), nitrogen replacement 3 times, heated in an oil bath at 80°C overnight, TLC detected that the starting compound 172-2 disappeared. Isopropanol was spin-dried, extracted with EA and ammonia water in sequence, the EA phase was taken, washed with saturated brine, citric acid, dried over anhydrous sodium sulfate, and spin-dried through the column to obtain intermediate 172-3, a white solid (3.0g, The purity is 78%). LC-MS: [M+H]...
Embodiment 2
[0197] Embodiment 2: post-processing of raw materials
[0198] Referring to Example 1, 11.1 g of Compound A was prepared. Add 20 mL of acetone, reflux at 65° C. (under nitrogen protection) for 2.0 h, spin dry the acetone directly, and vacuum-dry at 40° C. for 12 h. NMR shows that about 1% acetone remains. It was vacuum dried again at 80°C for 12 hours, and nuclear magnetic resonance showed that acetone remained. Take 5.3g of it and dry it under vacuum again at 80°C for 12h. NMR shows that there is still acetone residue. This batch of product was added to acetonitrile (16mL), refluxed at 85°C (nitrogen protection) for 2.0h, directly spin-dried the acetonitrile, and then vacuum-dried at 80°C for 12h, nuclear magnetic resonance showed that it was qualified and there was no residue, and 5.2g was put into storage. The purity of the product is 99.29%, and it is in the form of white powder.
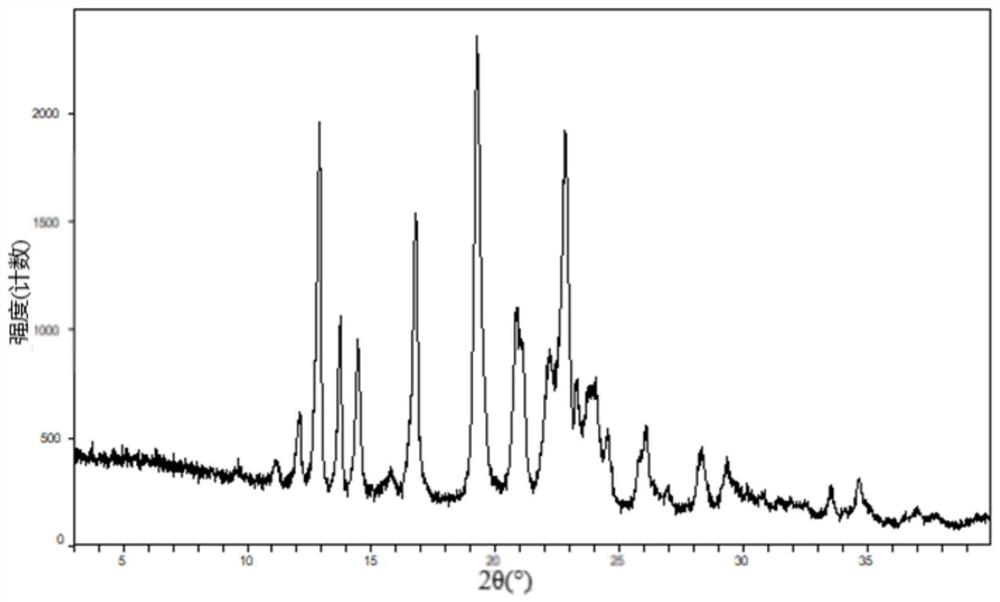
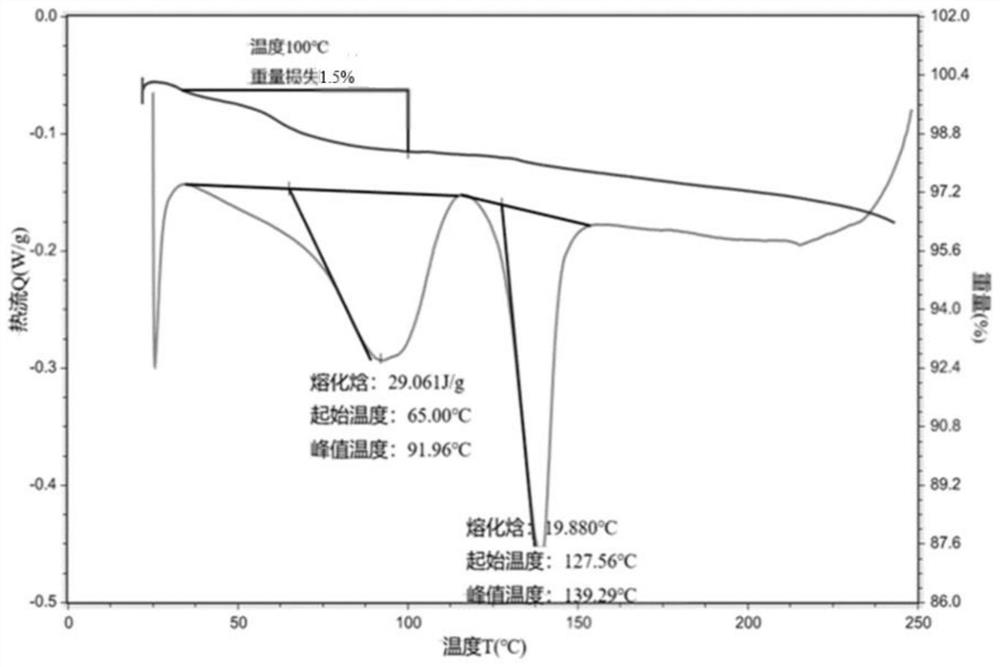
[0199] The results of PLM and XRPD show that the raw material is an irregular crystal with...
Embodiment 3
[0200] Embodiment 3: Preparation and characterization of the amorphous compound of formula A
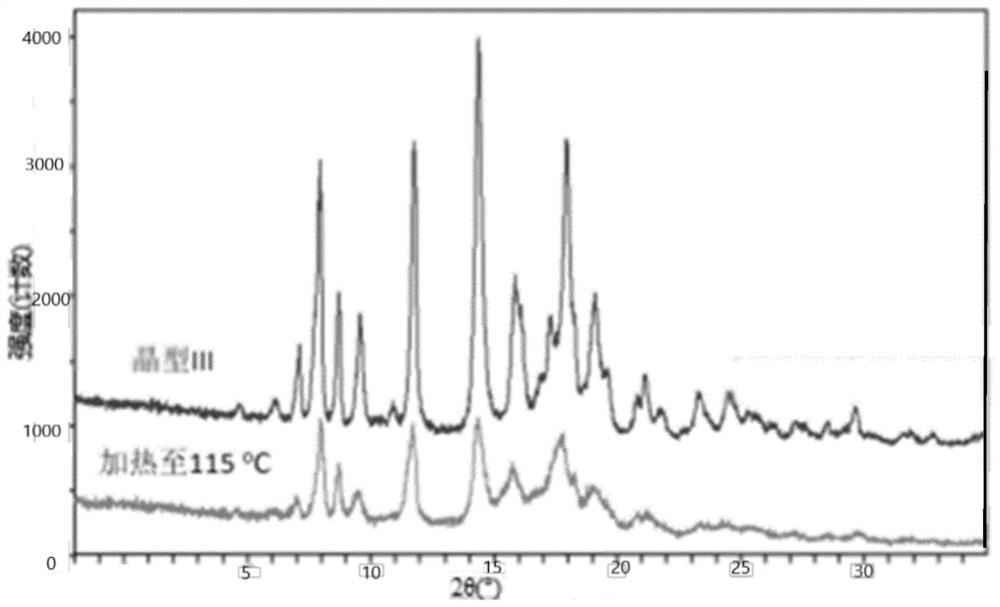
[0201] 3.1 Compound A was dissolved in a certain amount of THF, and concentrated to dryness under reduced pressure to obtain an amorphous sample. XRPD characterization see attached Figure 37 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com