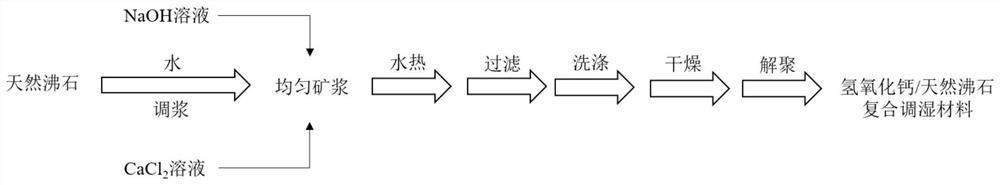
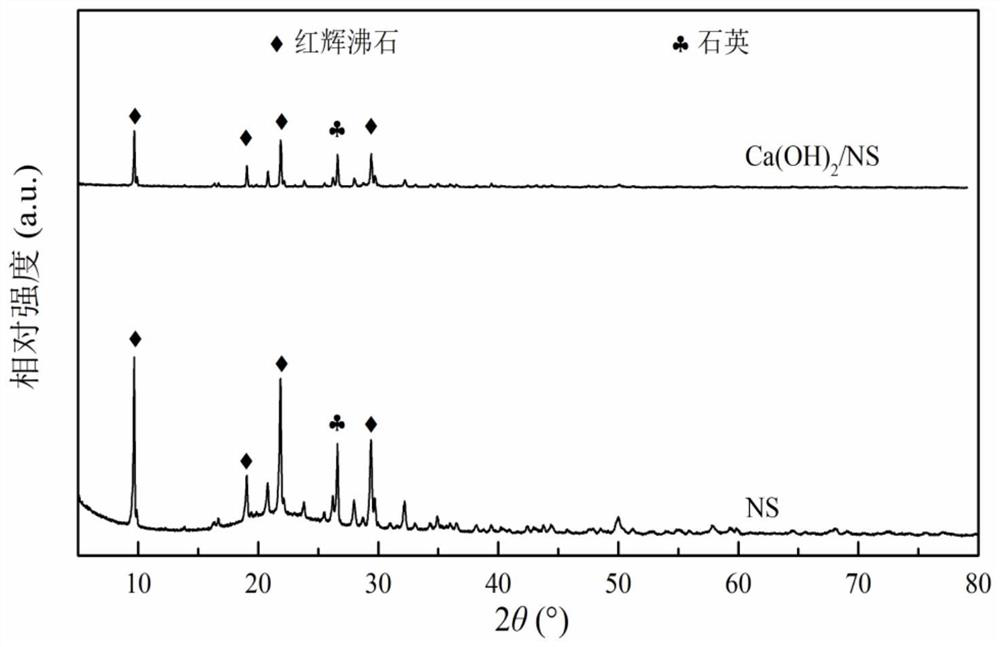
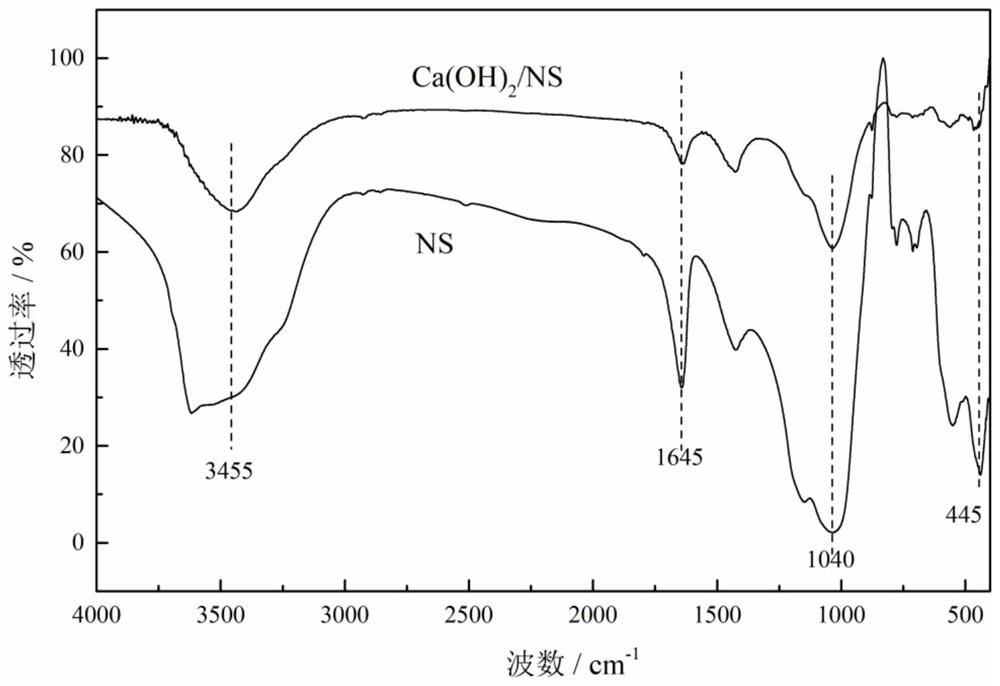
Calcium hydroxide/natural zeolite composite humidifying material and preparation method thereof
A technology of natural zeolite and humidity control materials, applied in chemical instruments and methods, silicon compounds, crystalline aluminosilicate zeolites, etc., to achieve the effects of optimization of pore structure and surface properties, process energy saving, green environmental protection, and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) First take by weighing 100g of natural staphilite powder (the powder fineness is D 97 =80 μm, the whiteness of the powder (blue light whiteness, the same below) is 89%, XRD quantitative analysis shows that the mineral content of hematilite in natural hematilite powder is 94%. The sample is marked as NS) and adds in the three-neck flask of 500ml; And predetermined Ca(OH) 2 The coating amount is 10%;
[0028] (2) According to the natural hematite powder quality (100g) and predetermined Ca(OH) 2 Coating amount (10%), calculate NaOH and CaCl 2 The reaction produces Ca(OH) 2 The theoretical mass is 10g, and according to NaOH and CaCl 2 The reaction produces Ca(OH) 2 Calculate the required NaOH and dihydrate CaCl by the molar ratio of the reaction 2 The masses are 10.81g and 19.86g respectively, and the corresponding masses of NaOH and dihydrate CaCl are weighed 2 ;
[0029] (3) According to the natural hematite powder quality (100g) taken by weighing, the calcula...
Embodiment 2
[0035] (1) First take by weighing 100g of natural clinoptilolite powder (the powder fineness is D 97 =100 μm, the whiteness of the powder is 78%, XRD quantitative analysis shows that the clinoptilolite mineral content in the natural clinoptilolite powder is 85%. The sample is marked as NC) and adds in the three-neck flask of 1000ml; And predetermined Ca(OH) 2 The coating amount is 20%;
[0036] (2) According to the natural clinoptilolite powder quality (100g) and predetermined Ca(OH) 2 Coating amount (20%), calculate NaOH and CaCl 2 The reaction produces Ca(OH) 2 The theoretical mass is 20g, and according to NaOH and CaCl 2 The reaction produces Ca(OH) 2 Calculate the required NaOH and dihydrate CaCl by the molar ratio of the reaction 2 The quality of each is 21.62g and 39.73g, and the corresponding quality of NaOH and dihydrate CaCl 2 ;
[0037] (3) According to the natural clinoptilolite powder quality (100g) taken by weighing, the calculated Ca(OH) 2 Theoretical ma...
Embodiment 3
[0043] (1) First weigh 100g of natural zeolite powder (the powder fineness is D 97 =75 μm, the whiteness of the powder is 75%, XRD quantitative analysis shows that the total mineral content of clinoptilolite and mordenite in the zeolite powder is 83%. The sample is marked as NZ) into a 1000ml three-neck flask; and predetermined Ca(OH) 2 The coating amount is 15%;
[0044] (2) According to the weighed natural zeolite powder quality (100g) and predetermined Ca(OH) 2 Coating amount (15%), calculated NaOH and CaCl 2 The reaction produces Ca(OH) 2 The theoretical mass is 15g, and according to NaOH and CaCl 2 The reaction produces Ca(OH) 2 Calculate the required NaOH and dihydrate CaCl by the molar ratio of the reaction 2 The quality of each is 16.22g and 29.80g, and the corresponding quality of NaOH and dihydrate CaCl 2 ;
[0045] (3) According to the weighed natural zeolite powder quality (100g), the calculated Ca(OH) 2 Theoretical mass (15g), according to liquid-solid ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| whiteness | aaaaa | aaaaa |
| whiteness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com