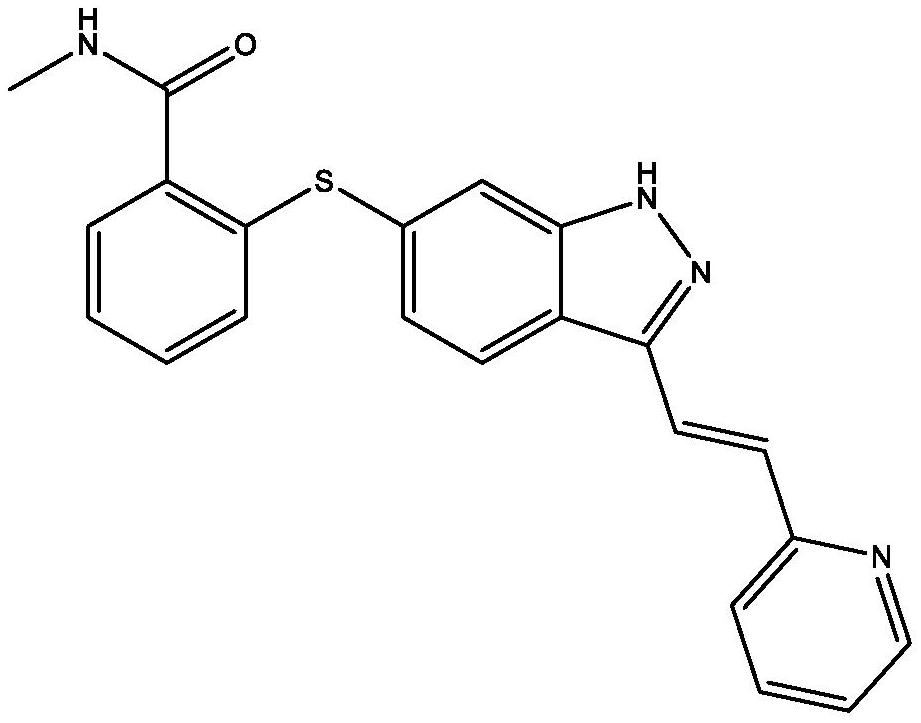
Axitinib crystal form
A technology of Axitinib and Ni crystal, which is applied in the field of Axitinib crystal form, can solve the problems of solubility, thermal stability, photostability, dissolution rate and bioavailability that cannot well meet the requirements of pharmaceutical preparations, and achieve Good light stability, improved drug bioavailability, and good solubility effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Add about 1g of axitinib to 30ml of glacial acetic acid, heat to 50°C and stir to dissolve, then add about 90ml of diethyl ether and continue to keep warm at 50°C and stir for 1 hour. crystallization; after the crystallization is completed, filter and dry to obtain the crystal form of axitinib with a yield of 97.8% and a purity of 99.92%.
Embodiment 2
[0046] Add about 1g of axitinib to 20ml of N,N-dimethylformamide, heat to 60°C and stir to dissolve, then add about 80ml of diethyl ether and continue to keep warm at 60°C and stir for 2 hours. After the reaction is over, the temperature of the reaction solution is cooled Stir and crystallize at 0-5°C; after the crystallization is completed, filter and dry to obtain the crystal form of axitinib with a yield of 97.2% and a purity of 99.93%.
Embodiment 3
[0048] Add about 1g of axitinib to 50ml of dimethyl sulfoxide, heat to 30°C and stir to dissolve, then add about 100ml of methyl ether and continue to keep warm at 30°C and stir for 1 hour. After the reaction is complete, the temperature of the reaction solution is cooled to -5 Stir and crystallize at ~0°C; after the crystallization is completed, filter and dry to obtain the crystalline form of axitinib with a yield of 96.5% and a purity of 99.90%.
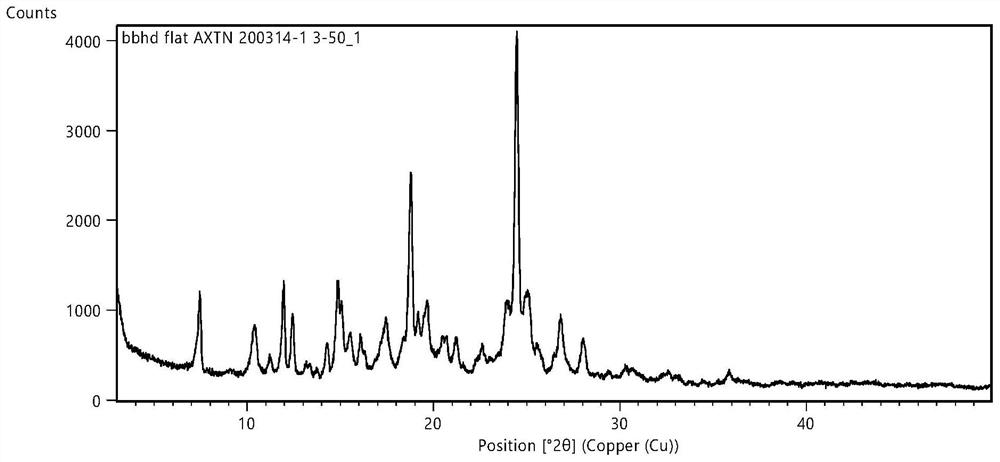
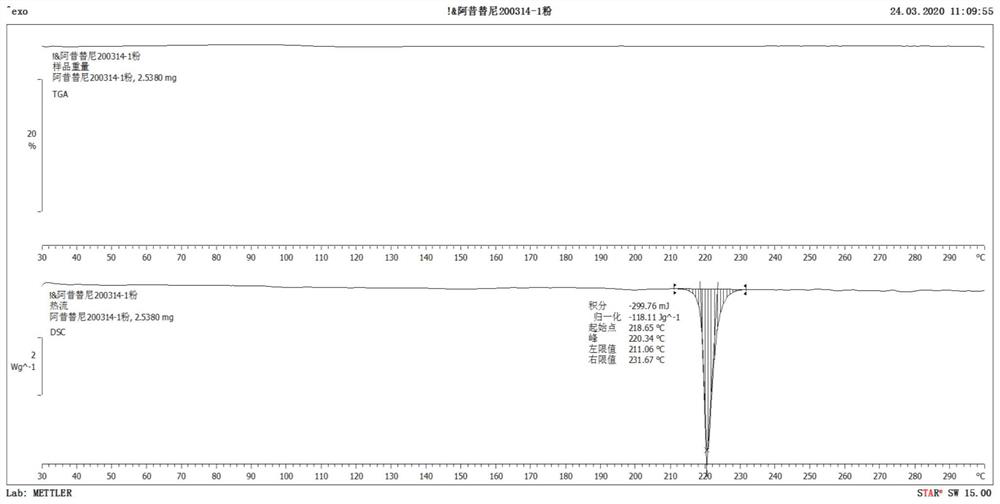
[0049] Axitinib crystal form characterization
[0050] The X-ray powder diffraction test instrument involved in the present invention and test condition: X-ray powder diffractometer: PANalytical EMPYREA; Cu-Kα; Sample table: flat panel; Incident light path: BBHD; Diffraction light path: PLXCEL; Voltage 45kv, electric current 40mA; Divergence slit: 1 / 4; Anti-scatter slit: 1; Solar slit: 0.04rad; Step size: 0.5s; Scanning range: 3~50°. The characteristic peaks in the corresponding X-ray secretion diffraction pattern (Cu-Kα) are det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com