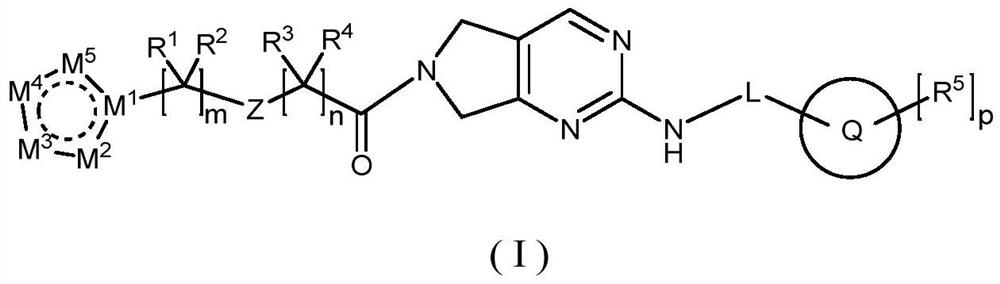
Pyrrolopyrimidine compound and application thereof
A compound and hydrate technology, applied in the field of pyrrolopyrimidine compounds and their preparation, pyrrolopyrimidine compounds, can solve the problem that patients with severe specific pulmonary fibrosis cannot benefit, cannot reverse pulmonary fibrosis, and cannot improve the quality of life of patients and other problems, to achieve the effects of excellent hepatic metabolism stability, inhibition of ATX enzyme activity, and excellent pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
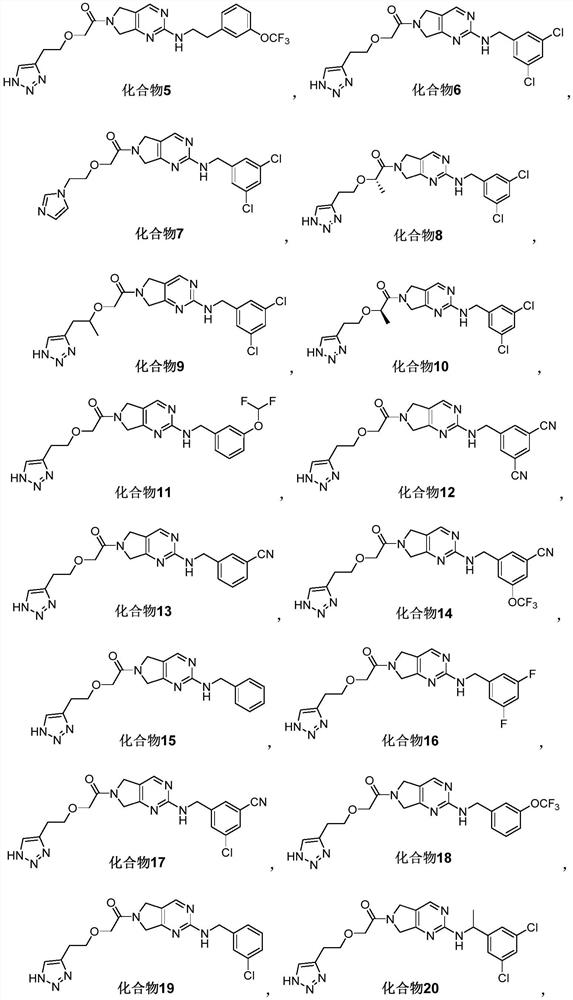
Examples
preparation example 1
[0121] Preparation Example 1: Synthesis of Intermediate B
[0122] 2-(2-(1H-1,2,3-Triazol-4-yl)ethoxy)acetic acid (Intermediate B)
[0123] The synthetic route of target compound intermediate B is as follows:
[0124]
[0125] The first step: Synthesis of 2-(butyl-3-yn-1-oxyl)methyl acetate (compound B-2)
[0126]The raw material 3-butynol (compound B-1) (2.8g, 0.4mol) was added to dry tetrahydrofuran (100mL), cooled to 0°C, 60% sodium hydrogen (2.4g, 0.6mmol) was added, and the After stirring at 0°C for 0.5h, the raw material 2-bromoacetate methyl ester (7.3g, 0.48mmol) was added to the reaction liquid, and the mixture was naturally warmed to room temperature, and stirred for 16h. Add water (100mL), extract with ethyl acetate (50mL×3), combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate, and the residue is separated and purified by silica gel column (petroleum ether: ethyl acetate (V / V )=5:1) Compound B-2 was obtained as a colorless liquid...
Embodiment 1
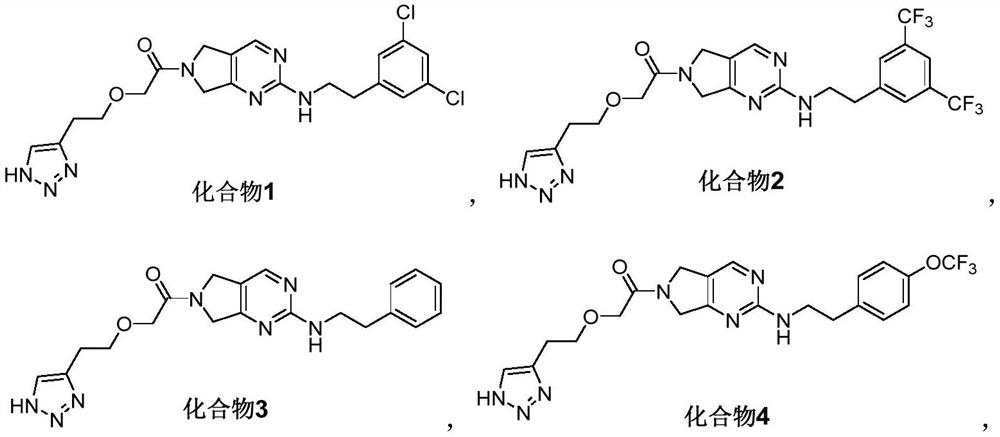
[0138] Embodiment 1: the synthesis of compound 1
[0139] 2-(2-(1H-1,2,3-triazol-4-yl)ethoxy)-1-(2-((3,5-dichlorophenethyl)amino)-5,7- Dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)ethan-1-one (compound 1)
[0140] The synthetic route of target compound 1 is as follows:
[0141]
[0142] The first step: 2-((3,5-dichlorophenethyl)amino)-5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidine-6-carboxylic acid tert-butyl ester (compound 1C )Synthesis
[0143] 2-Chloro-5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidine-6-carboxylic acid tert-butyl ester (0.75g, 2.93mmol) and 2-(3,5-dichlorobenzene Base) ethylamine (compound 1A) (0.557g, 2.93mmol), N,N-diisopropylethylamine (1.025mL, 5.87mmol) was dissolved in N-methylpyrrolidone (5mL), stirred at 80°C for 16 hours . Cool to room temperature, add distilled water (10 mL) to dilute, extract with ethyl acetate (10 mL×3), combine the organic phases, wash the organic phase with saturated brine (10 mL×2), separate the layers, and dry the organic phase with anhy...
Embodiment 2
[0151] Embodiment 2: the synthesis of compound 2
[0152] 2-(2-(1H-1,2,3-triazol-4-yl)ethoxy)-1-(2-((3,5-bis(trifluoromethyl)phenethyl)amino) Synthesis of -5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)ethan-1-one (compound 2)
[0153] The synthetic route of compound 2 is as follows:
[0154]
[0155] The first step: 2-((3,5-bis(trifluoromethyl)phenethyl)amino)-5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidine-6-carboxylic acid Synthesis of tert-butyl ester (Compound 2C)
[0156] 2-Chloro-5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidine-6-carboxylic acid tert-butyl ester (compound 2B) (0.145g, 0.567mmol) and 2-(3,5 - Bis(trifluoromethyl)phenyl)ethylamine hydrochloride (compound 2A) (200mg, 0.68mmol), diisopropylethylamine (0.297mL, 1.701mmol) dissolved in N-methylpyrrolidone (5mL) , stirred at 80°C for 16 hours. Cool to room temperature, add distilled water (10 mL) to dilute, extract with ethyl acetate (10 mL×3), combine the organic phases, wash the organic phase with saturated brine (10 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com