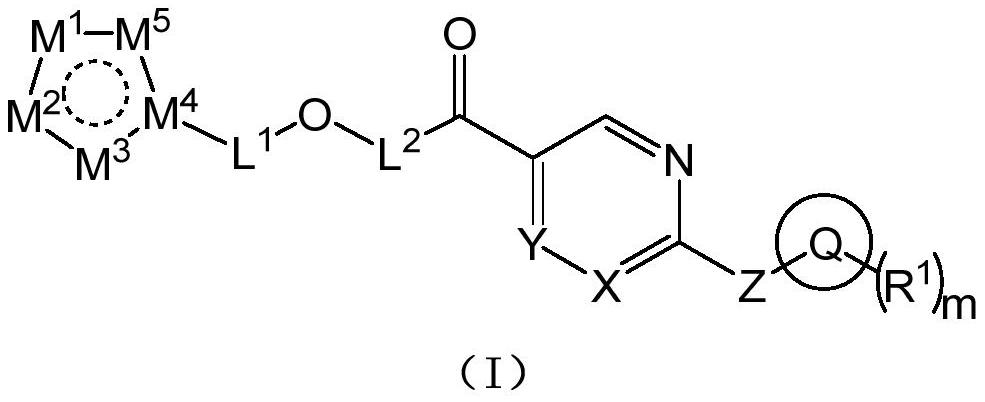
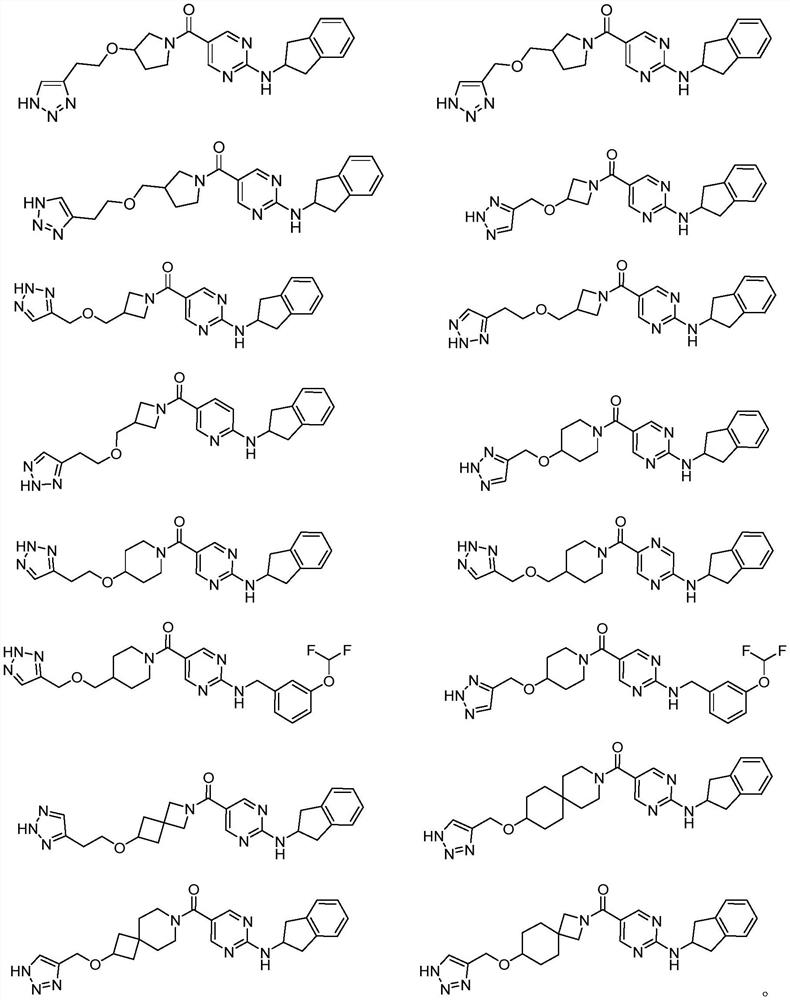
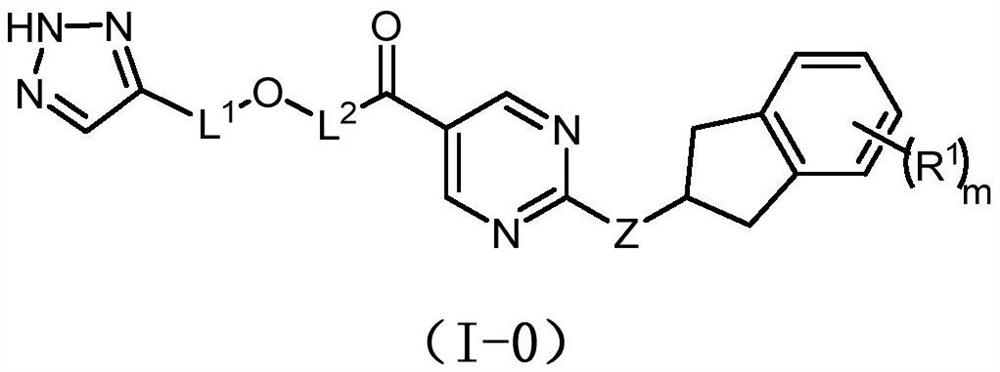
Pyrimidine compound and preparation method thereof
A technology of compounds and hydrates, applied in drug combination, organic chemistry, digestive system, etc., can solve the problems that patients with severe specific pulmonary fibrosis cannot benefit, affect the quality of life, and cannot improve the quality of life of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0150] Preparation Example 1: Preparation of Intermediate A
[0151] 2-((2,3-Dihydro-1H-inden-2-yl)amino)pyrimidine-5-carboxylic acid (Intermediate A)
[0152] 2-((2,3-dihydro-1H-inden-2-yl)amino)pyrimidine-5-carboxylic acid (Intermediate A)
[0153]
[0154] The synthetic route of intermediate A is as follows:
[0155]
[0156] Dissolve 2-chloropyrimidine-5-carboxylic acid (2g, 12.61mmol) in N-methylpyrrolidone (10mL), add 2-aminoindane hydrochloride (2.57g, 15.14mmol), N,N-diiso Propylethylamine (8.15g, 63.1mmol) was heated to 100°C for 24h. The reaction solution was evaporated to remove the solvent under the oil pump, ethyl acetate (30 mL) was added to the residue to disperse, filtered, the filter cake was beaten with water (30 mL), filtered, and air-dried at 50°C for 3 hours to obtain a gray solid 2-(2,3-di Hydrogen-1H-inden-2-ylamino)pyrimidine-5-carboxylic acid (2.4 g, 74.5% yield). Intermediate A used in the following examples can be obtained by referring to t...
preparation example 2
[0158] Preparation Example 2: Preparation of Intermediate B
[0159] 4-(((1H-1,2,3-triazol-4-yl)methoxy)methyl)piperidine hydrochloride (Intermediate B)
[0160]4-(((1H-1,2,3-triazol-4-yl)methoxy)methyl)piperidine hydrochloride (Intermediate B)
[0161]
[0162] The synthetic route of intermediate B is as follows:
[0163]
[0164] The first step: the synthesis of tert-butyl 4-((prop-2-yn-1-yloxy)methyl)piperidine-1-carboxylate (2)
[0165] tert-butyl 4-((prop-2-yn-1-yloxy)methyl)piperidine-1-carboxylate
[0166]
[0167] At 0°C, tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate (150 g, 697 mmol) was added to THF (1000 mL), then sodium hydride (33.4 g, 836 mmol, 60% purity ), stirred at room temperature for 1 h, then cooled to 0° C., slowly added 3-bromopropyne (104 g, 871 mmol) dropwise, and stirred at room temperature for 8 h after the addition was completed. After the reaction was completed, the reaction solution was poured into saturated aqueous ammonium ch...
Embodiment 1
[0178] Embodiment 1: the preparation of target compound I-1
[0179] (4-(((2H-1,2,3-triazol-4-yl)methoxy)methyl)piperidin-1-yl)(2-((2,3-dihydro-1H-indene -2-yl)amino)pyrimidin-5-yl)methanone (target compound I-1)
[0180] (4-(((2H-1,2,3-triazol-4-yl)methoxy)methyl)piperidin-1-yl)(2-((2,3-dihydro-1H-inden-2-yl)a mino)pyrimidin-5-yl)methanone
[0181]
[0182] The synthetic route of target compound 1-1 is as follows:
[0183]
[0184] The first step: the synthesis of tert-butyl 4-((prop-2-yn-1-yloxy)methyl)piperidine-1-carboxylate (I-1B)
[0185] tert-butyl4-((prop-2-yn-1-yloxy)methyl)piperidine-1-carboxylate
[0186]
[0187] Dissolve tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate (I-1A) (2g, 9.29mmol) in tetrahydrofuran (20mL), cool to 0°C, add sodium hydride (409mg, 10.22mmol , 60%), followed by dropwise addition of 3-bromopropyne (1.66 g, 14 mmol), and reacted at room temperature for 18 hours after the addition was completed. Add water (50mL) to quench, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com