Culture medium for CAR-T (Chimeric Antigen Receptor T cell) culture and application thereof
A technology of cell culture and culture medium, applied in the field of cell engineering, which can solve the problems of difficult specific target development, high recurrence rate of CAR-T technology, and easy exhaustion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
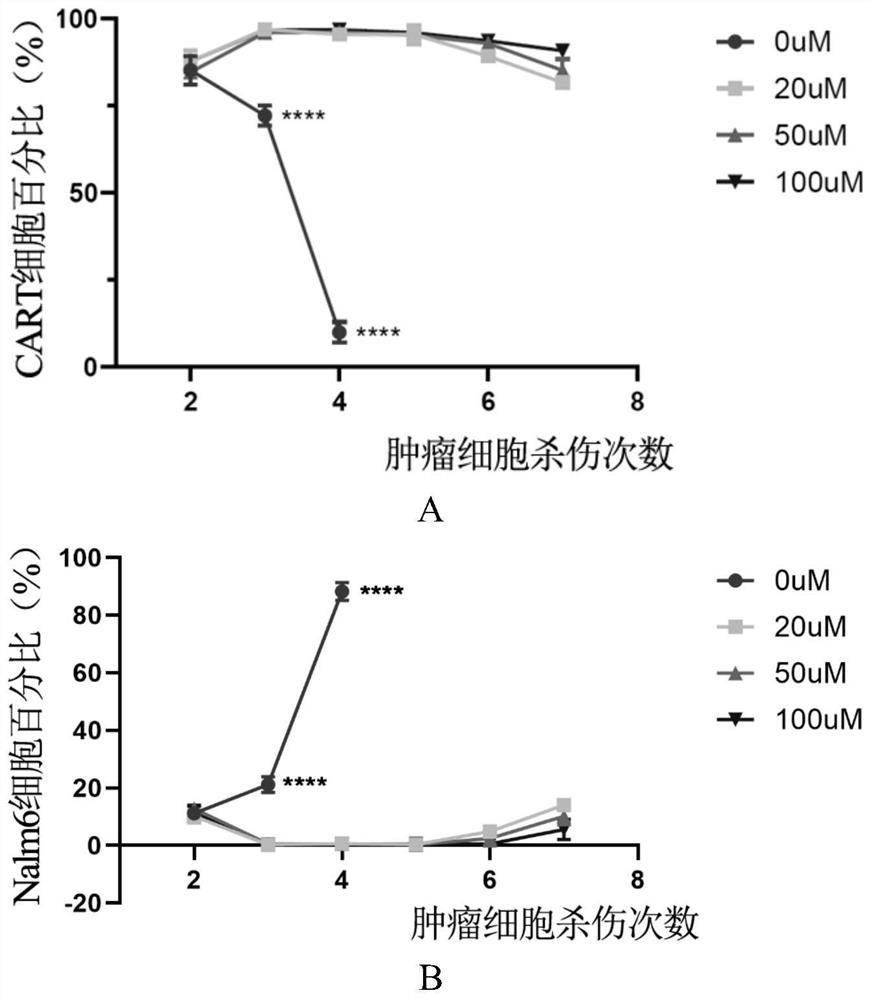
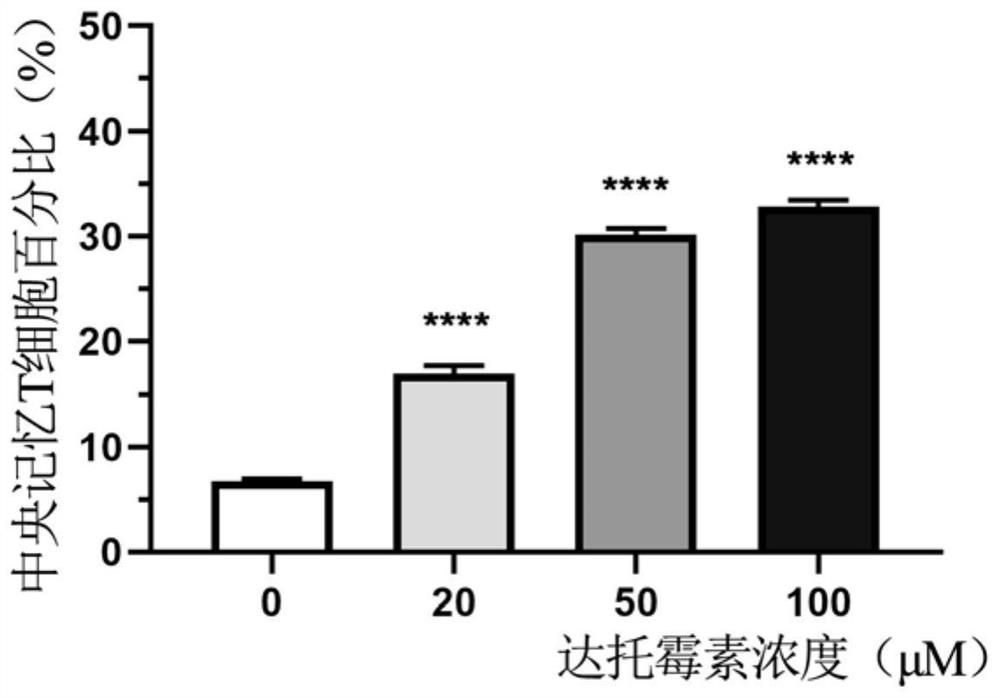
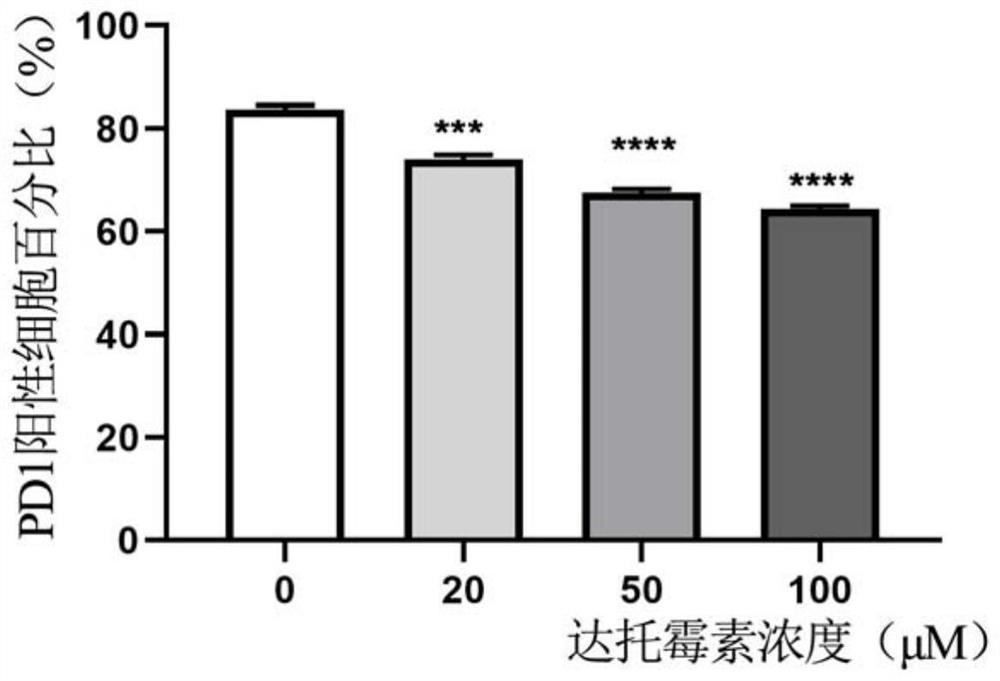
[0023] In the first aspect, the present invention provides a method for preparing D-CAR-T cells, the method comprising culturing conventional CAR-T cells under the treatment of daptomycin to obtain D-CAR-T cells, the D-CAR-T cells -CAR-T cells have higher killing ability and resistance to exhaustion.
[0024] According to the present invention, the adding period of daptomycin can be selected within a wide range. Preferably, the daptomycin is added continuously on the 6th-12th day of CAR-T cell culture, for example, 6th day, 9th day, 12th day.
[0025] According to the present invention, the time during which the CAR-T cells are cultured in daptomycin can also be selected within a wide range. Preferably, when the CAR-T cells are cultured in daptomycin for 48 hours, the CAR-T function can be significantly improved. More preferably, the CAR-T cells are cultured for a long time under daptomycin treatment, and the obtained D-CAR-T cells can more effectively promote the function o...
Embodiment 1
[0048] Embodiment 1: virus preparation
[0049] 1. Cultivate 293T cells with DMEM complete medium, wherein the DMEM complete medium includes DMEM (High Glucose) medium, 10% FBS by volume, 100 U / ml penicillin, and 100 μg / ml streptomycin. When the 293T density reached 60%-70%, the medium was changed, and 5ml of new DMEM complete medium was added, and the next step was carried out after culturing for 30min.
[0050] 2. Configure the public system of plasmids. The specification is to add 7.5 μg of the target plasmid (CD28z), 5.625 μg of pMD2.G plasmid, 1.875 μg of pMD2.G plasmid, 45 μl of PEI solution, and 200 μl of DMEM (High Glucose) medium into each 10 cm culture dish. Configure the DNA mixture in the order of DMEM (High Glucose) medium, plasmid, and PEI
[0051] 3. After standing still for 20 minutes, take the public system according to the required volume of each dish and evenly drop it into the petri dish, shake it crosswise for 2-3 times, and then put it into a 37°C incuba...
Embodiment 2
[0058] Example 2: Preparation of CAR-T
[0059] 1. Take 10ml of peripheral blood from healthy adults into EDTA-containing blood collection tubes, transfer the blood to a 50ml centrifuge tube with a dropper, add an equal volume of PBS solution to the blood, and mix well;
[0060] 2. With the ratio of blood volume: Ficoll lymphocyte separation liquid volume = 1:1, first add the separation liquid into a new 15mL centrifuge tube, and then gently add the blood mixed with PBS with a dropper;
[0061] 3. Centrifuge, set the rotation speed to 400g, the duration to 25min, and adjust the parameters to increase by 4 and decrease by 0;
[0062] 4. Use a pipette gun to suck up the buffy coat layer composed of peripheral blood mononuclear cells in the middle of the centrifuge tube, and suck it into a new centrifuge tube;
[0063] 5. Dilute to 15ml with PBS, wash, centrifuge at 300g for 7 minutes, and the parameters are 9 up to 9 down;
[0064] 6. Discard the supernatant after centrifugati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com