Preparation method of 6-azaindole
An azaindole, nitrogen technology, applied in the field of intermediate synthesis, can solve the problems of harsh anhydrous and oxygen-free conditions, unsuitable for industrialization, etc., and achieves the effects of simple reaction treatment, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
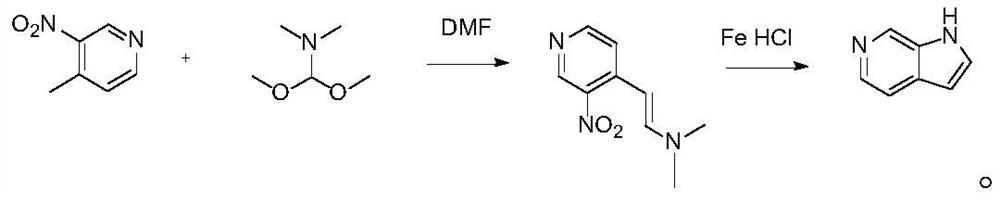
[0020] A preparation method of 6-azaindole, comprising the following steps:
[0021] Step 1. First prepare 4-(2-dimethylamino)ethylene-5-nitropyridine: add dry 290mL DMF to a 1L round bottom flask, then add 27.6g and 200mmol of 4-methyl-3- Nitropyridine and 2.76g of potassium carbonate, 26.6mL and 2.2 equivalents of dimethylformamide dimethyl acetal were added thereto under stirring; the mixture was heated to 85-95°C under a nitrogen atmosphere and stirred for 6 hours; the reaction When finished, the reaction was cooled to room temperature and the solution was poured into 500-700 mL of water; the dark orange-red suspension was filtered under vacuum, and the solid was collected and dried overnight in a vacuum oven to obtain 36.3 g of compound 4 with a yield of 94%. -(2-Dimethylamino)ethylene-5-nitropyridine;
[0022] Step 2. Dissolve 38.6g and 200mmol of the intermediate 4-(2-dimethylamino)ethylene-5-nitropyridine in 50% ethanol, add 3.0g and 600mmol of iron powder, and add th...
Embodiment 2
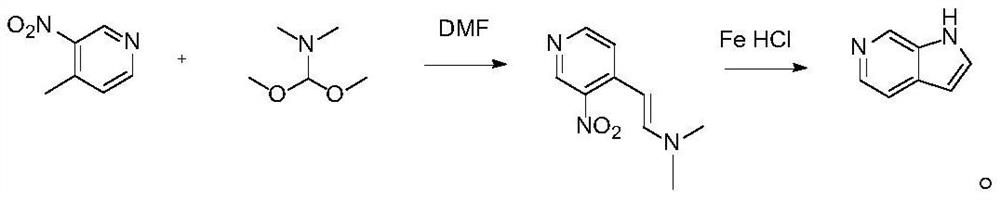
[0032] A preparation method of 6-azaindole, comprising the following steps:
[0033] Step 1. First prepare 4-(2-dimethylamino)ethylene-5-nitropyridine: add dry 290mL DMF to a 1L round bottom flask, then add 27.6g and 200mmol of 4-methyl-3- Nitropyridine and 2.76g of potassium carbonate, 26.6mL and 2.2 equivalents of dimethylformamide dimethyl acetal were added thereto under stirring; the mixture was heated to 85-95°C under a nitrogen atmosphere and stirred for 6 hours; the reaction When finished, the reaction was cooled to room temperature and the solution was poured into 500-700 mL of water; the dark orange-red suspension was filtered under vacuum, and the solid was collected and dried overnight in a vacuum oven to obtain 36.3 g of compound 4 with a yield of 94%. -(2-Dimethylamino)ethylene-5-nitropyridine;
[0034] Step 2. Dissolve 38.6g and 200mmol of the intermediate 4-(2-dimethylamino)ethylene-5-nitropyridine in 50% ethanol, add 3.0g and 600mmol of iron powder, and add th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com