Pharmaceutical dosage form comprising metformin and calcium citrate
A pharmaceutical dosage form, metformin technology, applied in the treatment of type 2 diabetes, metformin field, can solve problems such as affecting patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0187] first test
[0188] Three similar tablet blends were prepared, each containing metformin hydrochloride, a spray-dried formulation of vitamin B12 (available at Nutritional Products), binders and calcium salts. Thus, three different calcium salts were used respectively: calcium carbonate, calcium phosphate and tricalcium dicitrate tetrahydrate. In all three cases, capping was observed. Therefore, none of the three tablet blends of the first trial could be successfully compressed into tablets.
[0189] second test
[0190] Similar to the first trial, in the second trial three tablet mixtures were prepared. However, this time, granulated metformin (Metformin with a DC of 92.6%, granulated, with magnesium stearate as lubricant, available at Vistin Pharma) was used instead of non-granulated metformin hydrochloride. In the second experiment, no capping was observed regardless of which calcium salt was used.
[0191] Example 1 illustrates the preferred use of granulated ...
Embodiment 2
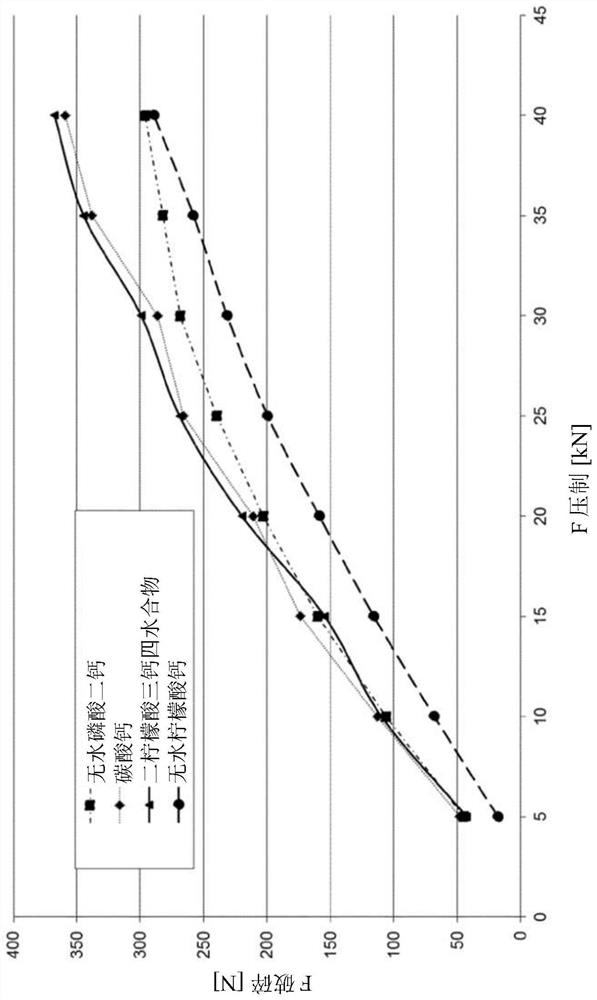
[0193] In Example 2, four similar tablet blends were prepared, each comprising a DC of 92.6% granulated metformin, a spray-dried formulation of vitamin B12 (available at Nutritional Products), Aerosol 200 as flow agent, magnesium stearate as lubricant, SMCC90 (available at JRS Pharma), and calcium salt. Therefore, four different types of calcium salts were tested: calcium carbonate (95MD, available at Particle Dynamics), dicalcium phosphate anhydrous (DiCafos A150, anhydrous, available at Budenheim), tricalcium dicitrate tetrahydrate (available at Merck) and calcium citrate anhydrous (available at Gadot).
[0194] For compressing the tablets, a single-punch tablet press (Korsch XP-1, available at Korsch, Berlin) was used.
[0195] All four tablet blends were successfully compressed into tablets regardless of the calcium salt used. Each of the tablets contains the same amount of metformin, vitamin B12 (spray-dried formulation), Ca 2+ (100mg / tablet), fluids and lubricants....
Embodiment 3
[0200] To further increase tablet hardness, four similar tablet blends were prepared as shown in Table 1 below:
[0201]
[0202] 1 Tricalcium dicitrate tetrahydrate (available at Merck)
[0203] 2 Calcium Carbonate (95MD, available at Particle Dynamics)
[0204] PH102 (available at FMC Biopolymer)
[0205] SMCC90 (available at JRS Pharma)
[0206] Table 1
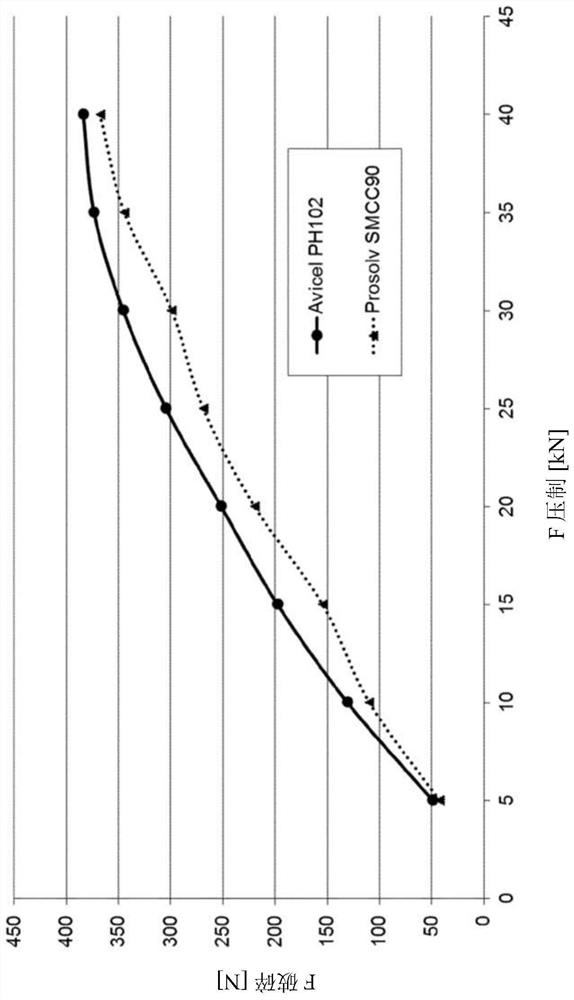
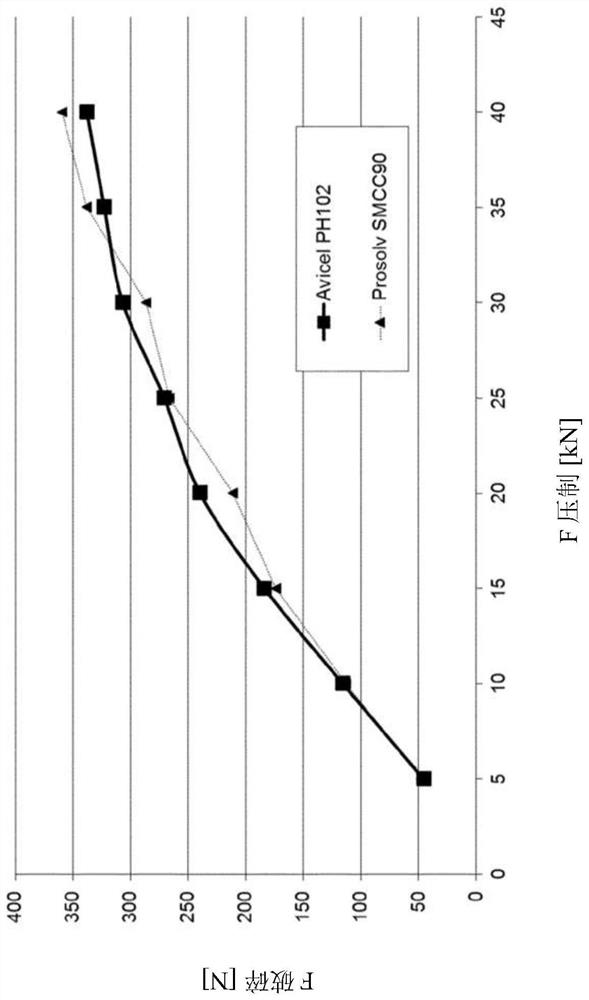
[0207] Similar to Example 2, the four tablet blends were compressed into tablets. Tablet hardness is then measured. result in Figure 2a and Figure 2b shown in .
[0208] Figure 2a shows the compression curve of the tablet containing as Ca 2+ source of tricalcium dicitrate tetrahydrate and as a binder PH102(3a) or SMCC90 (3a').
[0209] Figure 2b shows the compression curve of the tablet containing as Ca 2+ source of calcium carbonate and as a binder PH102(3b) or SMCC90 (3b').
[0210] Example 3 shows that by using microcrystalline cellulose as a binder, the hardness of tablets comprising m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com